Articles:
4-hexen-1-ol, 5-methyl-2-(1-methylethenyl)-, (2R)-
Notes:
Constit. of French lavender oil
| CAS Number: | 498-16-8 | 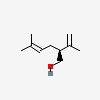 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 21090-68-6 | |
| FDA UNII: | T2QB7QHN63 | |
| Nikkaji Web: | J15.209I | |
| Beilstein Number: | 1722732 | |
| XlogP3-AA: | 3.00 (est) | |
| Molecular Weight: | 154.25266000 | |
| Formula: | C10 H18 O | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
| EFSA/JECFA Comments: | Register name to be changed to (R)-(-)-Lavandulol (EFFA, 2007b). | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| DG SANTE Food Flavourings: | 02.170 (R)-(-)-lavandulol |
Physical Properties:
| Appearance: | colorless to pale yellow clear liquid (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.87700 to 0.88300 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 7.298 to 7.347 |
| Refractive Index: | 1.46700 to 1.47300 @ 20.00 °C. |
| Boiling Point: | 229.00 to 230.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.013000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 191.00 °F. TCC ( 88.33 °C. ) |
| logP (o/w): | 2.665 (est) |
| Soluble in: | |
| alcohol | |
| water, very slightly | |
| water, 253.2 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Description: at 10.00 % in dipropylene glycol. | herbal |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| Lavandulol |
| Chemical Sources Association |
| Need This Item for Flavor/Food?: You can contact the CSA |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-mouse LD50 > 5000 mg/kg 4/10 mice died after a dose of 5000 mg/kg. (Moreno et al., 1982) oral-mouse LDLo 5000 mg/kg Food and Chemical Toxicology. Vol. 30, Pg. 55S, 1992. | |
| Dermal Toxicity: | |
|
skin-guinea pig LD50 > 5000 mg/kg Food and Chemical Toxicology. Vol. 30, Pg. 55S, 1992. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for (-)-lavandulol usage levels up to: | |||
| 2.0000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 0.012 (μg/capita/day) | ||
| Modified Theoretical Added Maximum Daily Intake (mTAMDI): | 3900 (μg/person/day) | ||
| Threshold of Concern: | 1800 (μg/person/day) | ||
| Structure Class: | I | ||
| Food categories according to Commission Regulation EC No. 1565/2000 (EC, 2000) in FGE.06 (EFSA, 2002a). According to the Industry the "normal" use is defined as the average of reported usages and "maximum use" is defined as the 95th percentile of reported usages (EFSA, 2002i). | |||
| Note: mg/kg = 0.001/1000 = 0.000001 = 1/1000000 = ppm. | |||
| average usage mg/kg | maximum usage mg/kg | ||
| Dairy products, excluding products of category 02.0 (01.0): | 7.00000 | 35.00000 | |
| Fats and oils, and fat emulsions (type water-in-oil) (02.0): | 5.00000 | 25.00000 | |
| Edible ices, including sherbet and sorbet (03.0): | 10.00000 | 50.00000 | |
| Processed fruit (04.1): | 7.00000 | 35.00000 | |
| Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds (04.2): | - | - | |
| Confectionery (05.0): | 10.00000 | 50.00000 | |
| Chewing gum (05.0): | - | - | |
| Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery (06.0): | 5.00000 | 25.00000 | |
| Bakery wares (07.0): | 10.00000 | 50.00000 | |
| Meat and meat products, including poultry and game (08.0): | 2.00000 | 10.00000 | |
| Fish and fish products, including molluscs, crustaceans and echinoderms (MCE) (09.0): | 2.00000 | 10.00000 | |
| Eggs and egg products (10.0): | - | - | |
| Sweeteners, including honey (11.0): | - | - | |
| Salts, spices, soups, sauces, salads, protein products, etc. (12.0): | 5.00000 | 25.00000 | |
| Foodstuffs intended for particular nutritional uses (13.0): | 10.00000 | 50.00000 | |
| Non-alcoholic ("soft") beverages, excl. dairy products (14.1): | 5.00000 | 25.00000 | |
| Alcoholic beverages, incl. alcohol-free and low-alcoholic counterparts (14.2): | 10.00000 | 50.00000 | |
| Ready-to-eat savouries (15.0): | 20.00000 | 100.00000 | |
| Composite foods (e.g. casseroles, meat pies, mincemeat) - foods that could not be placed in categories 01.0 - 15.0 (16.0): | 5.00000 | 25.00000 | |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 6 (FGE.06): Straight-and branched-chain aliphatic unsatured primary alcohols, aldehydes, carboxylic acids, and esters from chemical groups 1 and 4 View page or View pdf | |
| Flavouring Group Evaluation 6, Revision 1 (FGE.06Rev1) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 62 (FGE.62) Consideration of linear and branched-chain aliphatic unsaturated, unconjugated alcohols, aldehydes, acids, and related esters evaluated by JECFA (61st meeting) structurally related to esters of branched- and straight-chain aliphatic saturated primary alcohols and of one secondary alcohol, and branched- and straight-chain unsaturated carboxylic acids evaluated by EFSA in FGE.05 (2005) and to straight- and branched-chain aliphatic unsaturated primary alcohols, aldehydes, carboxylic acids, and esters evaluated by EFSA in FGE.06 (2004) (Commission Regulation (EC) No 1565/2000 of 18 July 2000) View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 06, Revision 2 (FGE.06Rev2): Straight- and branched-chain aliphatic unsaturated primary alcohols, aldehydes, carboxylic acids, and esters from chemical groups 1 and 4 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 06, Revision 3 (FGE.06Rev3): Straight- and branched-chain aliphatic unsaturated primary alcohols, aldehydes, carboxylic acids, and esters from chemical groups 1 and 4 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 06, Revision 4 (FGE.06Rev4): Straight- and branched-chain aliphatic unsaturated primary alcohols, aldehydes, carboxylic acids and esters from chemical groups 1, 3 and 4 View page or View pdf | |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 5464156 |
| National Institute of Allergy and Infectious Diseases: | Data |
| (2R)-5-methyl-2-prop-1-en-2-ylhex-4-en-1-ol | |
| Chemidplus: | 0000498168 |
References:
| Leffingwell: | Chirality or Article |
| (2R)-5-methyl-2-prop-1-en-2-ylhex-4-en-1-ol | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 498-16-8 |
| Pubchem (cid): | 5464156 |
| Pubchem (sid): | 135024331 |
| Flavornet: | 498-16-8 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| HMDB (The Human Metabolome Database): | HMDB36041 |
| FooDB: | FDB014861 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| herbal | FR | |
| lavender | FR |
Occurrence (nature, food, other): note
| lavender oil Search Trop Picture | |
| peppermint leaf Search Trop Picture | |
| rosemary plant Search Trop Picture |
Synonyms:
| 4- | hexen-1-ol, 2-isopropenyl-5-methyl-, (-)- (8CI) |
| 4- | hexen-1-ol, 5-methyl-2-(1-methylethenyl)-, (2R)- |
| 4- | hexen-1-ol, 5-methyl-2-(1-methylethenyl)-, (R)- |
| (R)- | lavandulol |
| (R)-(-)- | lavandulol |
| (R)-5- | methyl-2-(1-methyl ethenyl)-4-hexen-1-ol |
| (theta)-5- | methyl-2-(1-methyl ethenyl)-4-hexen-1-ol |
| (R)-5- | methyl-2-(1-methylethenyl)-4-hexen-1-ol |
| (2R)-5- | methyl-2-(1-methylethenyl)hex-4-en-1-ol |
| (2R)-5- | methyl-2-(prop-1-en-2-yl)hex-4-en-1-ol |
| (2R)-5- | methyl-2-prop-1-en-2-ylhex-4-en-1-ol |
| (-)-2-iso | propenyl-5-methyl-4-hexen-1-ol |
| 2-iso | propyl pentyl-5-methyl-4-hexen-1-ol |

