Articles:
p-menth-8-en-3-ol
Notes:
Various stereoisomers occur in essential oils. Flavouring agent
| CAS Number: | 7786-67-6 | 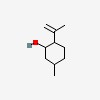 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 232-102-8 | |
| FDA UNII: | Search | |
| Nikkaji Web: | J36.816D | |
| XlogP3-AA: | 3.00 (est) | |
| Molecular Weight: | 154.25266000 | |
| Formula: | C10 H18 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| FEMA Number: | 2962 isopulegol |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 89-79-2 ; ISOPULEGOL |
| FDA Regulation: | |
| FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION Subpart F--Flavoring Agents and Related Substances Sec. 172.515 Synthetic flavoring substances and adjuvants. | |
Physical Properties:
| Appearance: | colorless clear liquid (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 197.00 to 198.00 °C. @ 760.00 mm Hg (est) |
| Vapor Pressure: | 0.099000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 173.00 °F. TCC ( 78.30 °C. ) (est) |
| logP (o/w): | 2.724 (est) |
| Soluble in: | |
| alcohol | |
| water, 308.6 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Type: minty | |
| Odor Strength: | medium , recommend smelling in a 10.00 % solution or less |
| Substantivity: | 28 hour(s) at 100.00 % |
| minty cooling medicinal woody green grassy herbal | |
| Odor Description: at 10.00 % in dipropylene glycol. | minty cooling medicinal woody green grassy herbal Luebke, William tgsc, (1985) |
| minty cooling medicinal woody green herbal | |
| Odor Description: | Minty, cooling, medicinal, woody with a green herbaceous undernote Mosciano, Gerard P&F 15, No. 6, 35, (1990) |
| Flavor Type: minty | |
| minty cooling herbal peppermint | |
| Taste Description: at 30.00 ppm. | Minty cooling, herbaceous peppermint nuance Mosciano, Gerard P&F 15, No. 6, 35, (1990) |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
fragrance |
Suppliers:
| Berjé |
| isoPulegol |
| Media |
| BOC Sciences |
| For experimental / research use only. |
| p-Menth-8-en-3-ol |
| Citrus and Allied Essences |
| isoPulegol Extra FCC |
| Market Report |
| CJ Latta & Associates |
| ISOPULEGOL |
| Coompo |
| For experimental / research use only. |
| isoPulegol from Plants ≥98%
Odor: characteristic Use: Isopulegol is a monoterpene alcohol intermediate in the preparation of (-)-menthol and it is present in the essential oils of various plants. This work presents behavioral effects of isopulegol in animal models of open field, elevated plus maze (EPM), rota rod, hole board, barbiturate-induced sleeping time, tail suspension and forced swimming tests in mice. Isopulegol was administered intraperitoneally to male mice at single doses of 25 and 50 mg/kg, while diazepam 1 or 2 mg/kg and imipramine 10 or 30 mg/kg were used as standard drugs. The results showed that, similar to diazepam (1 mg/kg), both doses of isopulegol significantly modified all the observed parameters in the EPM test, without alter the general motor activity in the open field test. In the same way, both doses of isopulegol increased the number of head dips in the hole-board test. Forced swimming and tail suspension tests showed that isopulegol (25 and 50 mg/kg) was able to induce a significant increase in the immobility time, in opposite to imipramine, a recognized antidepressant drug. There was a decrease in the sleep latency time and prolongation of the pentobarbital-induced sleeping time with both doses of Isopulegol. Different from diazepam (2 mg/kg), isopulegol (25 e 50 mg/kg) had no effect on the motor coordination of animals in the rota rod test. These results showed that isopulegol presented depressant- and anxiolytic-like effects.
The anticonvulsant and bioprotective effects of isopulegol against PTZ-induced convulsions are possibly related to positive modulation of benzodiazepine-sensitive GABAA receptors and to antioxidant properties. |
| Diffusions Aromatiques |
| isoPULEGOL |
| Global Essence |
| isoPulegol |
| Moellhausen |
| isoPULEGOL |
| Penta International |
| L-ISOPULEGOL |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| isoPulegol |
| Seqens |
| isoPulegol, Kosher |
| Som Extracts |
| ISO PULEGOL |
| Synerzine |
| ISOPULEGOL, SUM OF ISOMERS |
| TCI AMERICA |
| For experimental / research use only. |
| isoPulegol >90.0%(GC) |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 22 - Harmful if swallowed. R 36/38 - Irritating to skin and eyes. S 02 - Keep out of the reach of children. S 20/21 - When using do not eat, drink or smoke. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 37/39 - Wear suitable gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 1030 ul/kg Food and Cosmetics Toxicology. Vol. 13, Pg. 823, 1975. | |
| Dermal Toxicity: | |
|
skin-rabbit LD50 3 ml/kg Food and Cosmetics Toxicology. Vol. 13, Pg. 823, 1975. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| maximum skin levels for fine fragrances: | |||
| 0.0500 % and are based on the assumption that the fragrance mixture is used at 20% in a consumer product (IFRA Use Level Survey). (IFRA, 2004) | |||
| Recommendation for isopulegol usage levels up to: | |||
| 6.0000 % in the fragrance concentrate. | |||
| Dermal Systemic Exposure in Cosmetic Products: | |||
| 0.0007 mg/kg/day (IFRA, 2004) | |||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 3. Update in publication number(s): 22 | |||
| Click here to view publication 3 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | 14.00000 | 19.00000 | |
| beverages(nonalcoholic): | 50.00000 | 200.00000 | |
| beverages(alcoholic): | 50.00000 | 200.00000 | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | 500.00000 | 1000.00000 | |
| condiments / relishes: | - | - | |
| confectionery froastings: | 100.00000 | 500.00000 | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | 12.00000 | 15.00000 | |
| fruit ices: | - | - | |
| gelatins / puddings: | 50.00000 | 200.00000 | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | 100.00000 | 500.00000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | 100.00000 | 500.00000 | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of alicyclic substances used as flavor ingredients. View pdf | |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 7786-67-6 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 24585 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 2 |
| 5-methyl-2-prop-1-en-2-ylcyclohexan-1-ol | |
| Chemidplus: | 0007786676 |
| RTECS: | OT0190000 for cas# 7786-67-6 |
References:
| 5-methyl-2-prop-1-en-2-ylcyclohexan-1-ol | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 7786-67-6 |
| Pubchem (cid): | 24585 |
| Pubchem (sid): | 135019731 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| HMDB (The Human Metabolome Database): | HMDB36077 |
| FooDB: | FDB014911 |
| Export Tariff Code: | 2905.22.5050 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
Potential Uses:
| apricot | FR | |
| caramel | FL | |
| cherry | FR | |
| citronella | FR | |
| eucalyptus oil replacer | FR | |
| geranium | FR | |
| herbal | FR | |
| lemongrass | FR | |
| mandarin | FR | |
| mint | FR | |
| oral care agents | ||
| peach | FR | |
| pennyroyal | FL/FR | |
| peppermint | FR | |
| plum | FR | |
| raspberry | FR | |
| rose leaf | FL/FR | |
| spearmint | FR | |
| tuberose | FR |
Occurrence (nature, food, other): note
| angelica seed oil CO2 extract @ 1.40% Data GC Search Trop Picture | |
| bergamot oil @ 0.000-0.004% Data GC Search Trop Picture | |
| cascarilla bark oil @ 0.05% Data GC Search Trop Picture | |
| eucalyptus camaldulensis dehn. leaf oil jerusalem @ 3.80% Data GC Search Trop Picture | |
| eucalyptus citriadora Search Trop Picture | |
| eucalyptus citriodora oil @ 3.41% Data GC Search Trop Picture | |
| eucalyptus globulus oil pakistan @ 0.20% Data GC Search Trop Picture | |
| geranium Search Trop Picture | |
| grapefruit oil c.p. @ 0.05% Data GC Search Trop Picture | |
| grapefruit oil c.p. @ 0.06% Data GC Search Trop Picture | |
| grapefruit oil c.p. italy @ 0.04% Data GC Search Trop Picture | |
| laurel leaf oil turkey @ 0.10% Data GC Search Trop Picture | |
| lemon verbena oil morocco @ 0.7% Data GC Search Trop Picture | |
| lemongrass Search Trop Picture | |
| lemongrass oil @ 0.26% Data GC Search Trop Picture | |
| lemongrass oil @ 1.23% Data GC Search Trop Picture | |
| lemongrass oil morocco @ 0.30% Data GC Search Trop Picture | |
| lime oil distilled peru @ 0.08% Data GC Search Trop Picture | |
| mandarin Search Trop Picture | |
| mandarin oil @ trace% Data GC Search Trop Picture | |
| mandarin oil italy @ trace% Data GC Search Trop Picture | |
| mint Search Trop Picture | |
| pennyroyal oil @ trace% Data GC Search Trop Picture | |
| petitgrain combava oil @ 0.32% Data GC Search Picture | |
| petitgrain grapefruit oil @ 0.60% Data GC Search Trop Picture | |
| petitgrain lime oil @ 0.10% Data GC Search Trop Picture | |
| petitgrain sweet oil @ 0.51% Data GC Search Picture | |
| pseudotsuga menziesii Search Trop Picture | |
| pteronia oil @ 0.10% Data GC Search Trop Picture | |
| rum Search PMC Picture | |
| sage oil england @ 0.10% Data GC Search Trop Picture | |
| wormseed oil spain @ 3.54% Data GC Search Trop Picture |
Synonyms:
| p- | menth-8-en-3-ol |
| para- | menth-8-en-3-ol |
| 8(9)-p- | menthen-3-ol |
| 8(9)-para- | menthen-3-ol |
| 5- | methyl-2-(1-methyl vinyl) cyclohexan-1-ol |
| 5- | methyl-2-(1-methylvinyl)cyclohexan-1-ol |
| 5- | methyl-2-prop-1-en-2-ylcyclohexan-1-ol |
| 1- | methyl-4-isopropenyl cyclohexan-3-ol |
| iso | pulegol extra FCC |







