Articles:
(-)-(Z)-trimethyl-8-methylene bicyclo-4-undecene
Notes:
Widespread in plants (Jasminum, Origanum and Pimpinella spp.)
| CAS Number: | 118-65-0 | 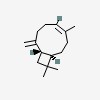 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 204-267-6 | |
| FDA UNII: | NRY8I0KNIR | |
| Nikkaji Web: | J5.303A | |
| MDL: | MFCD00135860 | |
| XlogP3-AA: | 4.40 (est) | |
| Molecular Weight: | 204.35628000 | |
| Formula: | C15 H24 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | pale yellow clear liquid to solid (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 266.00 to 268.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.013000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 220.00 °F. TCC ( 104.44 °C. ) |
| logP (o/w): | 6.416 (est) |
| Soluble in: | |
| alcohol | |
| water, 0.05011 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor Type: woody | |
| Odor Strength: | medium |
| woody spicy | |
| Odor Description: at 100.00 %. | woody spicy |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| (-)-isoCaryophyllene |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for isocaryophyllene usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for isocaryophyllene flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 118-65-0 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 5281522 |
| National Institute of Allergy and Infectious Diseases: | Data |
| (1R,4Z,9S)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene | |
| Chemidplus: | 0000118650 |
References:
| (1R,4Z,9S)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 118-65-0 |
| Pubchem (cid): | 5281522 |
| Pubchem (sid): | 135024438 |
| Flavornet: | 118-65-0 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C09691 |
| HMDB (The Human Metabolome Database): | HMDB61779 |
| FooDB: | FDB015737 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| balsam | FR | |
| clove | FR | |
| ginger | FR | |
| heather | FR | |
| herbal | FR | |
| spice | FR |
Occurrence (nature, food, other): note
| allspice plant Search Trop Picture | |
| angelica seed oil CO2 extract @ 2.00% Data GC Search Trop Picture | |
| anise plant Search Trop Picture | |
| anise seed oil star china @ 0.31% Data GC Search Trop Picture | |
| baccharis coridifolia oil argentina @ 34.30% Data GC Search Trop Picture | |
| basil plant Search Trop Picture | |
| calamintha nepeta (l.) savi subsp. glandulosa oil greece @ trace% Data GC Search Trop Picture | |
| chamomile sweet false chamomile plant Search Trop Picture | |
| cinnamon ceylon cinnamon bark Search Trop Picture | |
| clove plant Search Trop Picture | |
| croton flavens l. (welensali) leaf oil curacao @ 9.30% Data GC Search Trop Picture | |
| cumin seed oil Search Trop Picture | |
| hinoki leaf oil @ 1.65% Data GC Search Trop Picture | |
| hinoki root oil @ 0.55% Data GC Search Trop Picture | |
| hinoki wood oil @ 0.12% Data GC Search Trop Picture | |
| hyssop oil CO2 extract @ 2.5% Data GC Search Trop Picture | |
| lemon verbena oil morocco @ trace% Data GC Search Trop Picture | |
| lime oil distilled peru @ trace% Data GC Search Trop Picture | |
| orthodon dianthera maxim. oil vietnam @ 0.80% Data GC Search Trop Picture | |
| pepper black pepper fruit Search Trop Picture | |
| pepper black pepper fruit oil Search Trop Picture | |
| petitgrain combava oil @ trace% Data GC Search Picture | |
| rosemary Search Trop Picture | |
| sage oil spain @ 0.0-0.8% Data GC Search Trop Picture | |
| sage plant Search Trop Picture | |
| salvia plebeia oil @ 0.91% Data GC Search Trop Picture | |
| santolina oil @ 0.18% Data GC Search Trop Picture | |
| spearmint oil Search Trop Picture | |
| tagete oil zambia @ 2.20% Data GC Search Trop Picture | |
| turmeric root oil hydrodistilled @ 0.55% Data GC Search Trop Picture | |
| zingiber officinale root oil china @ 13.12% Data GC Search Trop Picture |
Synonyms:
| bicyclo(7.2.0)undec-4-ene, 4,11,11-trimethyl-8-methylene-, (1R,4Z,9S)- | |
| bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene-, (1R,4Z,9S)- | |
| (-)-(Z)-beta- | caryophyllene |
| (-)-iso | caryophyllene |
| gamma- | caryophyllene |
| iso | caryophyllene |
| (1R,9S)-8- | methylen-4,11,11-trimethylbicyclo[7.2.0]undec-4-ene |
| (-)-(Z)- | trimethyl-8-methylene bicyclo-4-undecene |
| (1R-(1R*,4Z,9S*))-4,11,11- | trimethyl-8-methylene bicyclo(7.2.0)undec-4-ene |
| (1R,4Z,9S)-4,11,11- | trimethyl-8-methylene bicyclo(7.2.0)undec-4-ene |
| (1R-(1R*,4Z,9S*))-4,11,11- | trimethyl-8-methylenebicyclo(7.2.0)undec-4-ene |
| (1R,4Z,9S)-4,11,11- | trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene |
| [1R-(1R*,4Z,9S*)]-4,11,11- | trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene |
| (1R,4Z,9S)-4,11,11- | trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene |
