Articles:
2-butenaldehyde
Notes:
None found
| CAS Number: | 4170-30-3 | 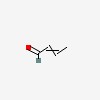 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 224-030-0 | |
| FDA UNII: | 9G72074TUW | |
| Beilstein Number: | 1209254 | |
| MDL: | MFCD00007003 | |
| XlogP3-AA: | 0.50 (est) | |
| Molecular Weight: | 70.09102000 | |
| Formula: | C4 H6 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | colorless to pale yellow clear liquid (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | -76.50 °C. @ 760.00 mm Hg |
| Boiling Point: | 104.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 30.000000 mmHg @ 20.00 °C. (est) |
| Flash Point: | 40.00 °F. TCC ( 4.60 °C. ) (est) |
| logP (o/w): | 0.771 (est) |
| Soluble in: | |
| water, 1.81E+05 mg/L @ 25 °C (exp) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Godavari Biorefineries |
| Crotonaldehyde |
| Sigma-Aldrich: Aldrich |
| For experimental / research use only. |
| Crotonaldehyde, mixture of cis and trans
ratio of cis- and trans-isomers (∼1:20), ≥99.5% (GC) |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intravenous-cat LDLo 30 mg/kg BLOOD: OTHER CHANGES Acta Pharmacologica et Toxicologica. Vol. 8, Pg. 275, 1952. oral-mouse LD50 104 mg/kg Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 26(8), Pg. 53, 1982. oral-rat LD50 80 mg/kg LUNGS, THORAX, OR RESPIRATION: CYANOSIS CARDIAC: PULSE RATE INCREASE WITHOUT FALL IN BP Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 62(3), Pg. 3, 1997. | |
| Dermal Toxicity: | |
|
skin-guinea pig LD50 30 uL/kg Journal of Industrial Hygiene and Toxicology. Vol. 26, Pg. 269, 1944. skin-rabbit LD50 380 uL/kg Union Carbide Data Sheet. Vol. 4/21/1967 | |
| Inhalation Toxicity: | |
|
inhalation-mouse LC50 580 mg/m3/2H Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 26(8), Pg. 53, 1982. inhalation-rat LC50 200 mg/m3/2H Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 26(8), Pg. 53, 1982. | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for 2-butenal usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for 2-butenal flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| NIOSH International Chemical Safety Cards: | search |
| NIOSH Pocket Guide: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 4170-30-3 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 20138 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| but-2-enal | |
| Chemidplus: | 0004170303 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | 4170-30-3 |
References:
| but-2-enal | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 4170-30-3 |
| Pubchem (cid): | 20138 |
| Pubchem (sid): | 134984616 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| KEGG (GenomeNet): | C19377 |
| HMDB (The Human Metabolome Database): | Search |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| apricot fruit Search Trop Picture | |
| chicory root oil @ 0.013% Data GC Search Trop Picture | |
| milk oxidized skim milk Search PMC Picture | |
| pear fruit Search Trop Picture |
Synonyms:
| but-2-enal | |
| 2- | buten-1-al |
| 2- | butenal propylene aldelhyde |
| 2- | butenaldehyde |
| crotonaldehyde | |
| crotonic aldehyde | |
| crotyl aldehyde | |
| 1- | formyl propene |
| 1- | formylpropene |
| beta- | methyl acrolein |
| methyl propenal |