Articles:
hydrolite 5 (Symrise)
Notes:
None found
| CAS Number: | 5343-92-0 | 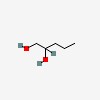 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 91049-43-3 | |
| ECHA EINECS - REACH Pre-Reg: | 226-285-3 | |
| FDA UNII: | 50C1307PZG | |
| Beilstein Number: | 1719151 | |
| MDL: | MFCD00010736 | |
| XlogP3-AA: | 0.20 (est) | |
| Molecular Weight: | 104.14904000 | |
| Formula: | C5 H12 O2 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: multi-functional cosmetic ingredient for skin and hair care
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | colorless clear liquid (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.96500 to 0.97500 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 8.030 to 8.113 |
| Refractive Index: | 1.43400 to 1.44400 @ 20.00 °C. |
| Boiling Point: | 198.00 to 199.00 °C. @ 760.00 mm Hg (est) |
| Vapor Pressure: | 0.057000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 220.00 °F. TCC ( 104.40 °C. ) (est) |
| logP (o/w): | 0.011 (est) |
| Soluble in: | |
| water, 7.664e+004 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
skin conditioning solvents |
Suppliers:
| BASF |
| 1,2-Pentanediol |
| BOC Sciences |
| For experimental / research use only. |
| 1,2-Pentanediol 95% |
| Connect Chemicals |
| Bio-pentylene glycol |
| ECSA Chemicals |
| PENTYLENE GLYCOL |
| ECSA TRADE THE MOST UPDATED FINANCIAL PUBLICATION ON THE WORLD OF CHEMISTRY |
| Kingyoung Bio Technical |
| Pentylene Glycol |
| Minasolve |
| MinaCare Pentiol (Pentylene glycol)
Odor: characteristic Use: Pentylene glycol is a very widely used moizturing agent, also showing interesting antimicrobial boosting properties.
Minasolve is a large producer of synthetic pentylene glycol of odouless and colourless quality for the cosmetic industry.
Its quality is recognized by small, medium and large companies all over the world, including the luxury brands. |
| Minasolve |
| MinaCare Pentiol Green ECOCERT (Pentylene Glycol)
Odor: characteristic Use: Pentylene Glycol is a very widely used moizturing agent, also showing interesting antimicrobial boosting properties.
Minasolve is a large producer of synthetic Pentylene Glycol of odouless and colourless quality for the cosmetic industry.
Its quality is recognized by small, medium and large companies all over the world, including the luxury brands. |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| 1,2-Pentanediol |
| Sigma-Aldrich: Aldrich |
| For experimental / research use only. |
| 1,2-Pentanediol 96% |
| Symrise |
| Hydrolite® 5 green
Odor: characteristic Use: Hydrolite® 5 green perfectly fits into the sustainability strategy of Symrise. Within the context of this strategy, the company emphasizes the importance of comprehensive transparency that benefits customers and end consumers. In 2018 the company won several awards for its exceptional global environmental protection efforts (Carbon Disclosure Project) and climate targets as well as for its environmental and social engagement (Ethibel Sustainability Index). The company currently assesses 80 percent of its main suppliers according to sustainability criteria. “Every time we use raw materials in our product development, we look at their sustainability as well as their social and environmental added value,” said Eder Ramos Global President Cosmetic Ingredients Division of Symrise. “With Hydrolite® 5 green we have reached a further milestone, because it is entirely made from byproducts of a renewable source, the sugar cane.” |
| Symrise |
| Hydrolite® 5
Odor: characteristic Use: Hydrolite® 5 is 1,2-pentanediol. Hydrolite® 5 is a colorless liquid with a charateristic intrinsic odor. Due to its chemical properties, Hydrolite® 5 is readily soluble in water and oil.
Hydrolite® 5 improves the water-binding capability of the skin and increases the degree of hydration. Hydrolite® 5 is also outstanding for its antimicrobial properties. |
| TCI AMERICA |
| For experimental / research use only. |
| 1,2-Pentanediol >98.0%(GC) |
| Vigon International |
| Hydrolite-5 (Pentylene Glycol) |
| Vigon International |
| HYDROLITE® 5 GREEN |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 12700 mg/kg BEHAVIORAL: EXCITEMENT BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: MUSCLE WEAKNESS Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 38(9), Pg. 14, 1973. oral-rabbit LD50 3700 mg/kg BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: EXCITEMENT BEHAVIORAL: MUSCLE WEAKNESS Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 38(9), Pg. 14, 1973. oral-mouse LD50 7400 mg/kg BEHAVIORAL: EXCITEMENT BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: MUSCLE WEAKNESS Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 38(9), Pg. 14, 1973. oral-guinea pig LD50 5200 mg/kg BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: EXCITEMENT BEHAVIORAL: MUSCLE WEAKNESS Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 38(9), Pg. 14, 1973. | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | multi-functional cosmetic ingredient for skin and hair care | ||
| Recommendation for pentylene glycol usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for pentylene glycol flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 5343-92-0 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 93000 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 1 |
| pentane-1,2-diol | |
| Chemidplus: | 0005343920 |
| RTECS: | SA0455000 for cas# 5343-92-0 |
References:
| pentane-1,2-diol | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 93000 |
| Pubchem (sid): | 135050899 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| HMDB (The Human Metabolome Database): | Search |
| Export Tariff Code: | 2905.39.9000 |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| hydrolite 5 (Symrise) | |
| hydrolite 5 green (Symrise) | |
| 1,2- | pentane diol |
| pentane-1,2-diol | |
| 1,2- | pentanediol |




