Articles:
D-8-p-menthene-1,2-epoxide
Notes:
Isol. from oil of Cymbopogon spp., orange (Citrus sinensis), Japanese pepper tree (Zanthoxylum piperitum) and others
| CAS Number: | 1195-92-2 | 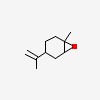 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 214-805-1 | |
| Nikkaji Web: | J27.205A | |
| Beilstein Number: | 111814 | |
| MDL: | MFCD00074770 | |
| XlogP3-AA: | 2.50 (est) | |
| Molecular Weight: | 152.23672000 | |
| Formula: | C10 H16 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 2145 D-8-p-menthene-1,2-epoxide |
| FEMA Number: | 4655 D-8-p-menthene-1,2-epoxide |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 203719-54-4 ; D-8-P-MENTHENE-1,2-EPOXIDE |
Physical Properties:
| Appearance: | colorless to pale yellow clear liquid (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.92600 to 0.93600 @ 20.00 °C. |
| Pounds per Gallon - (est).: | 7.714 to 7.798 |
| Refractive Index: | 1.46400 to 1.47400 @ 20.00 °C. |
| Boiling Point: | 198.00 °C. @ 760.00 mm Hg (est) |
| Vapor Pressure: | 0.515000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 150.00 °F. TCC ( 65.60 °C. ) (est) |
| logP (o/w): | 3.200 (est) |
| Soluble in: | |
| alcohol | |
| water, 137.2 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Type: citrus | |
| fresh clean citrus minty spearmint herbal | |
| Odor Description: Used in citrus and minty flavours: at 0.3-0.5ppm in beverages and dairy products, and up to 5ppm in sauces.Note: the epoxide can be lost in alcoholic and aqueous solutions, in acidic conditions. | fresh clean citrus minty spearmint herbal |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Achiewell |
| For experimental / research use only. |
| Limonene Oxide |
| BOC Sciences |
| For experimental / research use only. |
| Limonene-1,2-epoxide |
| Endeavour Specialty Chemicals |
| (+)-Limonene oxide, mixture of cis and trans |
| Speciality Chemical Product Groups |
| Penta International |
| LIMONENE OXIDE MIXED ISOMERS |
| R C Treatt & Co Ltd |
| (+)-Limonene Oxide
Kosher Odor: Sweet, citrus; very slight spearmint and herbaceous notes Flavor: citrus Used in citrus and minty flavours: at 0.3-0.5ppm in beverages and dairy products, and up to 5ppm in sauces.Note: the epoxide can be lost in alcoholic and aqueous solutions, in acidic conditions. |
| Robinson Brothers |
| (+)-Limonene oxide, mixture of cis and trans F&F |
| https://www.robinsonbrothers.uk/chemistry-competences |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| (+)-Limonene oxide, mixture of cis and trans, 97% |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intramuscular-mouse LD50 100 mg/kg Journal of Scientific and Industrial Research, Section C: Biological Sciences. Vol. 21, Pg. 342, 1962. oral-mouse LD50 2700 uL/kg Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 9, Pg. 518, 1978. | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 24 | |||
| Click here to view publication 24 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | - | |
| beverages(nonalcoholic): | 0.05000 | 0.50000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | 0.50000 | 5.00000 | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | - | |
| fruit ices: | - | - | |
| gelatins / puddings: | - | - | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | - | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | 0.05000 | 0.50000 | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 1195-92-2 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 91496 |
| National Institute of Allergy and Infectious Diseases: | Data |
| 6-methyl-3-prop-1-en-2-yl-7-oxabicyclo[4.1.0]heptane | |
| Chemidplus: | 0001195922 |
References:
| 6-methyl-3-prop-1-en-2-yl-7-oxabicyclo[4.1.0]heptane | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 1195-92-2 |
| Pubchem (cid): | 91496 |
| Pubchem (sid): | 135049028 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| UM BBD: | Search |
| KEGG (GenomeNet): | C07271 |
| HMDB (The Human Metabolome Database): | HMDB35158 |
| FooDB: | FDB013795 |
| Export Tariff Code: | 2932.99.0090 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
Potential Blenders and core components note
| For Odor | ||
| No odor group found for these | ||
| (R)- | limonene-10-ol | FL/FR |
| minty | ||
| laevo- | carvone | FL/FR |
| cis- | carvone-5,6-oxide | FL/FR |
| carvyl acetate | FL/FR | |
| dihydrocarveol | FL/FR | |
| trans-para- | menthan-2-one | FL/FR |
| tetrahydrocarvone | FL/FR | |
| For Flavor | ||
| No flavor group found for these | ||
| (R)- | limonene-10-ol | FL/FR |
| green | ||
| dihydrocarveol | FL/FR | |
| minty | ||
| laevo- | carvone | FL/FR |
| cis- | carvone-5,6-oxide | FL/FR |
| carvyl acetate | FL/FR | |
| trans-para- | menthan-2-one | FL/FR |
| tetrahydrocarvone | FL/FR | |
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| orange fruit Search Trop Picture | |
| pepper japanese pepper Search Trop Picture | |
| spearmint oil Search Trop Picture |
Synonyms:
| 7-oxa | bicyclo[4.1.0]heptane, 1-methyl-4- (1-methylethenyl)- |
| 7-oxa | bicyclo[4.1.0]heptane, 1-methyl-4-(1-methylethenyl)- |
| 1,2- | epoxylimonene |
| limonene 1,2-epoxide | |
| limonene 1,2-oxide | |
| limonene epoxide | |
| (cis+trans)-1,2-(+)- | limonene oxide |
| limonene oxide mixed isomers | |
| (+)- | limonene oxide, mixture of cis and trans |
| D-8-p- | menthene-1,2-epoxide |
| 6- | methyl-3-prop-1-en-2-yl-7-oxabicyclo[4.1.0]heptane |
| 1- | methyl-4-(1-methylethenyl)-7-oxabicyclo(4.1.0)heptane |
| 1- | methyl-4-(1-methylethenyl)-7-oxabicyclo[4.1.0]heptane |
| 1- | methyl-4-(1-methylvinyl)-7-oxabicyclo(4.1.0)heptane |
| 1- | methyl-4-(1-methylvinyl)-7-oxabicyclo[4.1.0]heptane |
| 1- | methyl-4-(prop-1-en-2-yl)-7-oxabicyclo[4.1.0]heptane |
| 4-iso | propenyl-1-methyl-7-oxabicyclo[4.1.0]heptan |
| 4-iso | propenyl-1-methyl-7-oxabicyclo[4.1.0]heptane |




