Articles:
ketohexamethylene
Notes:
Food flavourant. Present in various plant spp. e.g. Cistus ladaniferus (labdanum)
Cyclohexanone is a colorless oily liquid with an odor resembling acetone and peppermint. Cyclohexanone is occasionally found as a volatile component of human urine. . Biological fluids such as blood and urine have been shown to contain a large number of components, some of them volatiles (low boiling point) apparently present in all individuals, while others such are much more variable. In some cases differences up to an order of magnitude are observed. Although some of these changes may have dietary origins, others seem to be characteristic of the individual. Cyclohexanone is obtained through oxidation of cyclohexane or dehydrogenation of phenol. Approx. 95% of its manuf. is used for the production of nylon. Information on toxicity to human beings is fragmentary. Acute exposure is characterized by irritation of the eyes, nose, and throat. In two persons, drowsiness and renal impairment were found; Like cyclohexanol, cyclohexanone is not carcinogenic and is only moderately toxic, with a TLV of 25 ppm for the vapor. It is an irritant.; The great majority of cyclohexanone is consumed in the production of precursors to Nylon 66 and Nylon 6. About half of the world's supply is converted to adipic acid, one of two precursors for nylon 66. For this application, the KA oil (see above) is oxidized with nitric acid. The other half of the cyclohexanone supply is converted to the oxime. In the presence of sulfuric acid catalyst, the oxime rearranges to caprolactam, a precursor to nylon 6:; however, there were embryotoxic effects and influence on reproduction Cyclohexanone is well absorbed through the skin, respiratory tract, and alimentary tract. The main metabolic pathway leads to cyclohexanol, which is excreted in urine coupled with glucuronic acid. A high correlation was found between the concentration of cyclohexanone in the working environment and its concentration in urine. Cyclohexanone is formed from the hydrocarbons cyclohexane and 1-, 2-, and 3-hexanol. A patient's case report documents the development of anosmia (an olfactory disorder) and rhinitis caused by occupational exposure to organic solvents, including cyclohexanone (PMID: 10476412, 16925936, 16477465); however, these workers were also exposed to other compounds. Hepatic disorders were found in a group of workers exposed for over five years. In animals, cyclohexanone is characterized by relatively low acute toxicity (DL50 by intragastric administration is approx. 2 g/kg body wt.). Effects on the central nervous system (CNS) were found (narcosis), as well as irritation of the eyes and skin. Following multiple administration, effects were found in the CNS, liver, and kidneys as well as irritation of the conjunctiva. Mutagenic and genotoxic effects were found, but no teratogenic effects were detected
| CAS Number: | 108-94-1 | 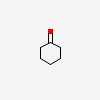 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 203-631-1 | |
| FDA UNII: | 5QOR3YM052 | |
| Nikkaji Web: | J2.872J | |
| Beilstein Number: | 0385735 | |
| MDL: | MFCD00001625 | |
| CoE Number: | 11047 | |
| XlogP3: | 0.80 (est) | |
| Molecular Weight: | 98.14490000 | |
| Formula: | C6 H10 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: food contact microporous polymeric filters
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 1100 cyclohexanone |
| DG SANTE Food Flavourings: | 07.148 cyclohexanone |
| FEMA Number: | 3909 cyclohexanone |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 108-94-1 ; CYCLOHEXANONE |
| FDA Regulation: | |
| FDA PART 177 -- INDIRECT FOOD ADDITIVES: POLYMERS Subpart C--Substances for Use Only as Components of Articles Intended for Repeated Use Sec. 177.2250 Filters, microporous polymeric. | |
Physical Properties:
| Appearance: | colorless clear liquid (est) |
| Assay: | 99.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.94700 to 0.95000 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 7.880 to 7.905 |
| Refractive Index: | 1.44700 to 1.45300 @ 20.00 °C. |
| Melting Point: | -47.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 154.00 to 156.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 4.330000 mmHg @ 25.00 °C. |
| Vapor Density: | 3.4 ( Air = 1 ) |
| Flash Point: | 116.00 °F. TCC ( 46.67 °C. ) |
| logP (o/w): | 0.810 |
| Soluble in: | |
| alcohol | |
| water, 2.408e+004 mg/L @ 25 °C (est) | |
| water, 2.50E+04 mg/L @ 25 °C (exp) | |
Organoleptic Properties:
| Odor Type: minty | |
| minty acetone | |
| Odor Description: at 1.00 % in dipropylene glycol. | minty acetone |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents solvents |
Suppliers:
| Covalent Chemical |
| Cyclohexanone |
| ECSA Chemicals |
| CYCLOHEXANONE 99,5% |
| ECSA TRADE THE MOST UPDATED FINANCIAL PUBLICATION ON THE WORLD OF CHEMISTRY |
| EMD Millipore |
| For experimental / research use only. |
| Cyclohexanone |
| Indenta Group |
| Cyclohexanone |
| Lluch Essence |
| CYCLOHEXANONE 99% |
| Penta International |
| CYCLOHEXANONE REAGENT ACS GRADE |
| Penta International |
| CYCLOHEXANONE |
| Rainbow Chemical |
| Cyclohexanone 99.8 |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| Cyclohexanone |
| Sigma-Aldrich |
| Cyclohexanone 99.8% |
| Certified Food Grade Products |
| Silver Fern Chemical |
| Cyclohexanone
Odor: characteristic Use: Cyclohexanone is mainly used in the production of Nylon as a precursor to Nylon 6 and Nylon 6,6. It can also be used in the electronic industry, adhesives, agriculture, and paints. |
| TCI AMERICA |
| For experimental / research use only. |
| Cyclohexanone >99.0%(GC) |
| Wedor Corporation |
| CYCLOHEXANONE |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 10 - Flammable. R 20 - Harmful by inhalation. R 36/37/38 - Irritating to eyes, respiratory system, and skin. S 02 - Keep out of the reach of children. S 25 - Avoid contact with eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 36/37/39 - Wear suitable clothing, gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 [sex: M,F] 1705 mg/kg (Kohli et al., 1967) oral-rat LD50 [sex: M,F] 1840 mg/kg (Deichmann & LeBlanc, 1943) oral-rat LD50 [sex: M,F] 1620 mg/kg (Smyth et al., 1969a) oral-rat LD50 [sex: M,F] 1800 mg/kg (Gupta et al., 1979) oral-mouse LD50 [sex: M,F] 2070 mg/kg (Gupta et al., 1979) gavage-rabbit LD50 [sex: M] 1600 mg/kg (Treon et al., 1943) intraperitoneal-rabbit LD50 [sex: M] 1540 mg/kg (Gupta et al., 1979) intraperitoneal-guinea pig LD50 [sex: M] 930 mg/kg (Price, 1951) intraperitoneal-guinea pig LDLo 760 mg/kg National Technical Information Service. Vol. AD-A066-307 oral-mammal (species unspecified) LD50 3000 mg/kg Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 32(10), Pg. 25, 1988. intraperitoneal-mouse LD50 1230 mg/kg GASTROINTESTINAL: OTHER CHANGES Toxicology and Applied Pharmacology. Vol. 49, Pg. 525, 1979. oral-mouse LD50 1400 mg/kg National Technical Information Service. Vol. AD-A066-307 intraperitoneal-rabbit LD50 1540 mg/kg GASTROINTESTINAL: OTHER CHANGES Toxicology and Applied Pharmacology. Vol. 49, Pg. 525, 1979. oral-rabbit LDLo 1600 mg/kg BEHAVIORAL: GENERAL ANESTHETIC Journal of Industrial Hygiene and Toxicology. Vol. 25, Pg. 199, 1943. intraperitoneal-rat LD50 1130 mg/kg GASTROINTESTINAL: OTHER CHANGES Toxicology and Applied Pharmacology. Vol. 49, Pg. 525, 1979. oral-rat LD50 1620 uL/kg American Industrial Hygiene Association Journal. Vol. 30, Pg. 470, 1969. intravenous-rat LDLo 568 mg/kg Toxicology and Applied Pharmacology. Vol. 37, Pg. 115, 1976. | |
| Dermal Toxicity: | |
|
skin-rabbit LD50 1 ml/kg American Industrial Hygiene Association Journal. Vol. 30, Pg. 470, 1969. subcutaneous-rat LD50 2170 mg/kg American Industrial Hygiene Association Journal. Vol. 30, Pg. 470, 1969. subcutaneous-frog LDLo 1900 mg/kg AUTONOMIC NERVOUS SYSTEM: OTHER (DIRECT) PARASYMPATHOMIMETIC BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 50, Pg. 199, 1903. subcutaneous-mouse LDLo 1300 mg/kg LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BEHAVIORAL: ATAXIA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 50, Pg. 199, 1903. subcutaneous-rat LD50 2170 mg/kg Journal of Industrial Hygiene and Toxicology. Vol. 25, Pg. 415, 1943. | |
| Inhalation Toxicity: | |
|
inhalation-rat LC50 8000 ppm/4H Raw Material Data Handbook, Vol.1: Organic Solvents, 1974. Vol. 1, Pg. 18, 1974. inhalation-mammal (species unspecified) LC50 25000 mg/m3 Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 32(10), Pg. 25, 1988. inhalation-guinea pig LCLo 400 ppm/4H National Technical Information Service. Vol. AD-A066-307 inhalation-human TCLo 75 ppm LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES SENSE ORGANS AND SPECIAL SENSES: CONJUNCTIVE IRRITATION: EYE SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION Journal of Industrial Hygiene and Toxicology. Vol. 25, Pg. 282, 1943. inhalation-mouse TCLo 19200 mg/m3/90 GASTROINTESTINAL: OTHER CHANGES Toxicology and Applied Pharmacology. Vol. 49, Pg. 525, 1979. | |
Safety in Use Information:
| Category: | food contact microporous polymeric filters | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 0.12 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 0.10 (μg/capita/day) | ||
| Structure Class: | II | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 19 | |||
| Click here to view publication 19 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | 6.00000 | 12.00000 | |
| beverages(nonalcoholic): | 2.50000 | 5.00000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | 100.00000 | 200.00000 | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | 2.00000 | 5.00000 | |
| fruit ices: | - | - | |
| gelatins / puddings: | - | - | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | - | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | 12.00000 | 25.00000 | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Flavouring Group Evaluation 51, (FGE.51)[1] - Consideration of alicyclic ketones and secondary alcohols and related esters evaluated by JECFA (59th meeting) and structurally related to alicyclic ketones, secondary alcohols and related esters evaluated by EFSA in FGE.09 (2004) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food View page or View pdf | |
| Flavouring Group Evaluation 9, Revision 1: (FGE.09 Rev1)[1] - Secondary alicyclic saturated and unsaturated alcohols, ketones and esters containing secondary alicyclic alcohols from chemical groups 8 and 30, and an ester of a phenol carboxylic acid from chemical group 25 - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food View page or View pdf | |
| Flavouring Group Evaluation 9, Revision 2 (FGE.09Rev2): Secondary alicyclic saturated and unsaturated alcohols, ketones and esters containing secondary alicyclic alcohols from chemical group 8 and 30, and an ester of a phenol derivative from chemical group 25 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 51, Revision 1 (FGE.51Rev1): Consideration of alicyclic ketones and secondary alcohols and related esters evaluated by the JECFA (59th meeting) structurally related to alicyclic ketones secondary alcohols and related esters in FGE.09Rev3 (2011) View page or View pdf | |
| Flavouring Group Evaluation 51, Revision 2 (FGE.51Rev2): Consideration of alicyclic ketones and secondary alcohols and related esters evaluated by JECFA (59th meeting) structurally related to alicyclic ketones secondary alcohols and related esters in FGE.09Rev6 (2015) View page or View pdf | |
| EPI System: | View |
| EPA-Iris: | IRIS |
| NIOSH International Chemical Safety Cards: | search |
| NIOSH Pocket Guide: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| Carcinogenic Potency Database: | Search |
| EPA Substance Registry Services (TSCA): | 108-94-1 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 7967 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 1915 |
| WGK Germany: | 1 |
| cyclohexanone | |
| Chemidplus: | 0000108941 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | 108-94-1 |
References:
| cyclohexanone | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 108-94-1 |
| Pubchem (cid): | 7967 |
| Pubchem (sid): | 134973338 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| FDA Indirect Additives used in Food Contact Substances: | View |
| CHEBI: | View |
| CHEMBL: | View |
| Metabolomics Database: | Search |
| UM BBD: | Search |
| KEGG (GenomeNet): | C00414 |
| HMDB (The Human Metabolome Database): | HMDB03315 |
| FooDB: | FDB003418 |
| Export Tariff Code: | 2914.22.1000 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| Formulations/Preparations: •technical: 99.8% •commercial: 89%; high purity: 99.5% | |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| basil oil Search Trop Picture | |
| brassica napus ssp. oleifera Search Trop Picture | |
| cistus ladaniferus Search Trop Picture | |
| musa spp. Search Trop Picture | |
| passiflora mucronata Search Trop Picture | |
| pepper bell pepper fruit Search Trop Picture | |
| tea green tea Search Trop Picture | |
| vriesea gladioliflora Search Trop Picture |
Synonyms:
| anon | |
| cyclohexyl ketone | |
| cycloyhexyl ketone | |
| keto | hexamethylene |
| hexanon | |
| hytrol O | |
| nadone | |
| pimelic ketone | |
| pimelin ketone | |
| sextone |




