| Info: | Cinnamaldehyde |
| Google Patents: | Use of cis-cinnamaldehyde as flavouring agent |
(2Z)-3-phenylacrylaldehyde
| CAS Number: | 57194-69-1 | 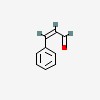 3D/inchi 3D/inchi
|
| FDA UNII: | W3HD274KF5 | |
| Nikkaji Web: | J918.664F | |
| XlogP3: | 1.90 (est) | |
| Molecular Weight: | 132.16196000 | |
| Formula: | C9 H8 O | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| Appearance: | pale yellow to yellow clear liquid (est) |
| Assay: | 94.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 245.00 to 246.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.027000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 160.00 °F. TCC ( 71.11 °C. ) |
| logP (o/w): | 2.123 (est) |
| Soluble in: | |
| alcohol | |
| water, very slightly | |
| water, 2150 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
| Odor Type: spicy | |
| Odor Strength: | medium , recommend smelling in a 10.00 % solution or less |
| spicy cinnamon | |
| Odor Description: at 10.00 % in dipropylene glycol. | spicy cinnamon |
| Flavor Type: spicy | |
| cinnamon | |
| Taste Description: | cinnamon |
| Odor and/or flavor descriptions from others (if found). | |
| None found |
| None found |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 21/41 - Harmful in contact with skin, risk of serious damage to eyes. R 38 - Irritating to skin. R 43 - May cause sensitisation by skin contact. S 02 - Keep out of the reach of children. S 24 - Avoid contact with skin. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 36/37/39 - Wear suitable clothing, gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
| Category: | flavor and fragrance agents | ||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| IFRA Critical Effect: | Sensitization | ||
| IFRA fragrance material specification: | |||
| The concentration of Cinnamic aldehyde in the finished cosmetic product should not exceed 0.1%. | |||
| View IFRA Standards Library for complete information. | |||
| Please review Amendment 49 IFRA documentation for complete information. | |||
| IFRA RESTRICTION LIMITS IN THE FINISHED PRODUCT (%): | |||
| Category 1: Products applied to the lips | |||
| 0.02 % | |||
| Category 2: Products applied to the axillae | |||
| 0.02 % | |||
| Category 3: Products applied to the face/body using fingertips | |||
| 0.05 % | |||
| Category 4: Products related to fine fragrance | |||
| 0.05 % | |||
| Category 5: Products applied to the face and body using the hands (palms), primarily leave-on | |||
| Category 5A: Body lotion products applied to the body using the hands (palms), primarily leave-on | |||
| 0.05 % | |||
| Category 5B: Face moisturizer products applied to the face using the hands (palms), primarily leave-on | |||
| Category 5C: Hand cream products applied to the hands using the hands (palms), primarily leave-on | |||
| Category 5D: Baby Creams, baby Oils and baby talc | |||
| Category 6: Products with oral and lip exposure | |||
| 0.40 % | |||
| Category 7: Products applied to the hair with some hand contact | |||
| Category 7A: Rinse-off products applied to the hair with some hand contact | |||
| 0.40 % | |||
| Category 7B: Leave-on products applied to the hair with some hand contact | |||
| Category 8: Products with significant anogenital exposure | |||
| 0.05 % | |||
| Category 9: Products with body and hand exposure, primarily rinse off | |||
| 0.05 % | |||
| Category 10: Household care products with mostly hand contact | |||
| Category 10A: Household care excluding aerosol products (excluding aerosol/spray products) | |||
| 0.05 % | |||
| Category 10B: Household aerosol/spray products | |||
| Category 11: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate | |||
| Category 11A: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate without UV exposure | |||
| See Note % | |||
| Category 11B: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate with potential UV exposure | |||
| Category 12: Products not intended for direct skin contact, minimal or insignificant transfer to skin | |||
| Notes: | |||
| IFRA FLAVOR REQUIREMENTS: | |||
Due to the possible ingestion of small amounts of fragrance ingredients from their use in products in Categories 1 and 6, materials must not only comply with IFRA Standards but must also be recognized as safe as a flavoring ingredient as defined by the IOFI Code of Practice (www.iofi.org). For more details see chapter 1 of the Guidance for the use of IFRA Standards. | |||
For this material, for pragmatic reasons, restrictive levels allowed by the QRA for certain categories but actually being higher than those already in place before applying the QRA, will temporarily not be implemented until the end of a 5 year monitoring phase. At the end of the 5 years the position will be reevaluated again. | |||
(1) IFRA would recommend that any material used to impart perfume or flavour in products intended for human ingestion should consist of ingredients that are in compliance with appropriate regulations for foods and food flavourings in the countries of planned distribution and, where these are lacking, with the recommendations laid down in the Code of Practice of IOFI (International Organisation of the Flavor Industry). Further information about IOFI can be found on its website (www.iofi.org). | |||
(2) Category 11 includes all non-skin contact or incidental skin contact products. Due to the negligible skin contact from these types of products there is no justification for a restriction of the concentration of this fragrance ingredient in the finished product. | |||
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 6428995 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| (Z)-3-phenylprop-2-enal | |
| Chemidplus: | 0057194691 |
| (Z)-3-phenylprop-2-enal | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 6428995 |
| Pubchem (sid): | 135157648 |
| Pherobase: | View |
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| HMDB (The Human Metabolome Database): | HMDB32072 |
| FooDB: | FDB008783 |
| Export Tariff Code: | 2912.29.5000 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| allspice | FR | |
| almond | FR | |
| apricot | FR | |
| aramis | ||
| balsam | FR | |
| banana | FR | |
| brut | ||
| bubble gum | FR | |
| butterscotch | FR | |
| canoe | ||
| carnation | FR | |
| cassia | FR | |
| catsup | FL | |
| cherry | FR | |
| cherry wild cherry | FL | |
| chocolate cacao | FL | |
| cinnabar | ||
| cinnamon | FR | |
| cola | FL | |
| cranberry | FR | |
| date | FR | |
| floral | FR | |
| florida soap | FR | |
| foliage | FR | |
| grenadine | FL | |
| halston | ||
| herbal | FR | |
| hyacinth | FR | |
| l'air du temps | ||
| l'origan | ||
| millefleurs | FR | |
| mint | FR | |
| myrrh | FR | |
| norell | ||
| old spice | ||
| opium | ||
| oriental | FR | |
| paco rabanne | ||
| patchouli | FR | |
| pierre cardin | ||
| plum | FR | |
| potpourri | FR | |
| raspberry | FR | |
| rose | FR | |
| sassafras | FR | |
| sensen | ||
| sesame | FL | |
| spice | FR | |
| strawberry | FR | |
| tonka bean | FR | |
| tuberose | FR | |
| valerian | FL/FR | |
| vanilla | FR | |
| windsor soap | FR | |
| youth dew |
| cinnamomum fragrans baillon leaf oil madagascar @ 0.90% Data GC Search Trop Picture | |
| cinnamon bark oil (cinnamomum zeylanicum blume) sri lauka @ 0.12-0.98% Data GC Search Trop Picture | |
| cinnamon ceylon cinnamon leaf Search Trop Picture | |
| cinnamon fruit oil india @ 0.2-0.3% Data GC Search Trop Picture |
| cis- | cinnamaldehyde |
| (Z)- | cinnamic aldehyde |
| cis- | cinnamic aldehyde |
| (2Z)-3- | phenylacrylaldehyde |
| (Z)-3- | phenylprop-2-enal |
| 2- | propenal, 3-phenyl-, (2Z)- |