Articles:
3,7,11-trimethyl-2,6,10-dodecatrienal
Notes:
None found
| CAS Number: | 19317-11-4 | 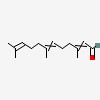 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 242-957-9 | |
| FDA UNII: | R265G157TQ | |
| Beilstein Number: | 1723427 | |
| MDL: | MFCD00038089 | |
| CoE Number: | 5148 | |
| XlogP3-AA: | 4.90 (est) | |
| Molecular Weight: | 220.35548000 | |
| Formula: | C15 H24 O | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
| EFSA/JECFA Comments: | Mixture of (Z)- and (E)-isomer for both C=C double bonds (EFFA, 2010). 10-15 % (2Z,6Z); 20-25 % (2E,6Z); 20-25% (2Z,6E); 40-50 % (2E,2E) (EFFA, 2013). | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 1228 3,7,11-trimethyl-2,6,10-dodecatrienal |
| DG SANTE Food Flavourings: | 05.148 farnesal |
| FEMA Number: | 4019 3,7,11-trimethyl-2,6,10-dodecatrienal |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 19317-11-4 ; FARNESAL |
Physical Properties:
| Appearance: | pale yellow to yellow clear liquid (est) |
| Assay: | 99.00 to 100.00 % sum of isomers |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.89000 to 0.90000 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 7.406 to 7.489 |
| Refractive Index: | 1.49400 to 1.50400 @ 20.00 °C. |
| Boiling Point: | 126.00 to 129.00 °C. @ 3.50 mm Hg |
| Boiling Point: | 198.00 to 201.00 °C. @ 8.00 mm Hg |
| Acid Value: | 3.00 max. KOH/g |
| Vapor Pressure: | 0.000187 mmHg @ 25.00 °C. (est) |
| Flash Point: | > 212.00 °F. TCC ( > 100.00 °C. ) |
| logP (o/w): | 5.013 (est) |
| Soluble in: | |
| alcohol | |
| water, 0.4278 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Description: at 100.00 %. | floral minty |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| FARNESAL ≥85% |
| Frinton Laboratories |
| For experimental / research use only. |
| Farnesal, Pract. |
| Parchem |
| farnesal |
| Penta International |
| FARNESAL |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| Farnesal |
| Sigma-Aldrich |
| Farnesal, mixture of isomers, ≥85% |
| Certified Food Grade Products |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xi - Irritant | |
|
R 36/37/38 - Irritating to eyes, respiratory system, and skin. S 02 - Keep out of the reach of children. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 36 - Wear suitable protective clothing. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| IFRA Critical Effect: | Dermal sensitization and systemic toxicity | ||
| IFRA: | View Standard | ||
| View IFRA Standards Library for complete information. | |||
| Please review Amendment 49 IFRA documentation for complete information. | |||
| IFRA RESTRICTION LIMITS IN THE FINISHED PRODUCT (%): | |||
| Category 1: Products applied to the lips | |||
| 0.11 % | |||
| Category 2: Products applied to the axillae | |||
| 0.032 % | |||
| Category 3: Products applied to the face/body using fingertips | |||
| 0.11 % | |||
| Category 4: Products related to fine fragrance | |||
| 0.60 % | |||
| Category 5: Products applied to the face and body using the hands (palms), primarily leave-on | |||
| Category 5A: Body lotion products applied to the body using the hands (palms), primarily leave-on | |||
| 0.15 % | |||
| Category 5B: Face moisturizer products applied to the face using the hands (palms), primarily leave-on | |||
| 0.15 % | |||
| Category 5C: Hand cream products applied to the hands using the hands (palms), primarily leave-on | |||
| 0.15 % | |||
| Category 5D: Baby Creams, baby Oils and baby talc | |||
| 0.051 % | |||
| Category 6: Products with oral and lip exposure | |||
| 0.11 % | |||
| Category 7: Products applied to the hair with some hand contact | |||
| Category 7A: Rinse-off products applied to the hair with some hand contact | |||
| 0.34 % | |||
| Category 7B: Leave-on products applied to the hair with some hand contact | |||
| 0.34 % | |||
| Category 8: Products with significant anogenital exposure | |||
| 0.051 % | |||
| Category 9: Products with body and hand exposure, primarily rinse off | |||
| 0.57 % | |||
| Category 10: Household care products with mostly hand contact | |||
| Category 10A: Household care excluding aerosol products (excluding aerosol/spray products) | |||
| 0.57 % | |||
| Category 10B: Household aerosol/spray products | |||
| 4.20 % | |||
| Category 11: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate | |||
| Category 11A: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate without UV exposure | |||
| 0.051 % | |||
| Category 11B: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate with potential UV exposure | |||
| 0.051 % | |||
| Category 12: Products not intended for direct skin contact, minimal or insignificant transfer to skin | |||
| No Restriction | |||
| Notes: | |||
| IFRA FLAVOR REQUIREMENTS: | |||
Due to the possible ingestion of small amounts of fragrance ingredients from their use in products in Categories 1 and 6, materials must not only comply with IFRA Standards but must also be recognized as safe as a flavoring ingredient as defined by the IOFI Code of Practice (www.iofi.org). For more details see chapter 1 of the Guidance for the use of IFRA Standards. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 0.49 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 0.20 (μg/capita/day) | ||
| Threshold of Concern: | 1800 (μg/person/day) | ||
| Structure Class: | I | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 20 | |||
| Click here to view publication 20 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | 2.00000 | 10.00000 | |
| beverages(nonalcoholic): | 0.10000 | 0.80000 | |
| beverages(alcoholic): | 0.30000 | 2.00000 | |
| breakfast cereal: | 0.10000 | 0.80000 | |
| cheese: | - | - | |
| chewing gum: | 2.00000 | 15.00000 | |
| condiments / relishes: | - | - | |
| confectionery froastings: | 0.30000 | 2.00000 | |
| egg products: | 0.30000 | 2.00000 | |
| fats / oils: | 0.30000 | 2.00000 | |
| fish products: | - | - | |
| frozen dairy: | 0.50000 | 6.00000 | |
| fruit ices: | 0.20000 | 2.10000 | |
| gelatins / puddings: | 0.20000 | 2.00000 | |
| granulated sugar: | - | - | |
| gravies: | 0.30000 | 2.00000 | |
| hard candy: | 0.50000 | 5.00000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | 0.10000 | 1.00000 | |
| jams / jellies: | 0.50000 | 5.00000 | |
| meat products: | - | - | |
| milk products: | 0.20000 | 2.10000 | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | 0.50000 | 5.00000 | |
| snack foods: | 0.50000 | 5.00000 | |
| soft candy: | 0.50000 | 5.00000 | |
| soups: | 0.10000 | 0.80000 | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Flavouring Group Evaluation 202: 3-Alkylated aliphatic acyclic alpha,beta-unsaturated aldehydes and precursors with or without additional double bonds from chemical subgroup 1.1.3 of FGE.19[1] View page or View pdf | |
| Flavouring Group Evaluation 72 (FGE.72): Consideration of aliphatic, branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters evaluated by the JECFA (61st meeting) structurally related to branched- and straight-chain unsaturated carboxylic acids. Esters of these and straight-chain aliphatic saturated alcohols evaluated by EFSA in FGE.05Rev2 (2010) View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 72, Revision 1 (FGE.72Rev1): Consideration of aliphatic, branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters evaluated by the JECFA (61st meeting) structurally related to branched- and straight-chain unsaturated carboxylic acids, esters of these and straight-chain aliphatic saturated alcohols evaluated by EFSA in FGE.05Rev2 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 72, Revision 2 (FGE.72Rev2): consideration of aliphatic, branched-chain saturated and unsaturated alcohols, aldehydes, acids and related esters evaluated by JECFA (61st, 68th and 69th meetings) and structurally related to flavouring substances in FGE.05Rev3 View page or View pdf | |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 19317-11-4 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 68150 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 2 |
| 3,7,11-trimethyldodeca-2,6,10-trienal | |
| Chemidplus: | 0019317114 |
References:
| 3,7,11-trimethyldodeca-2,6,10-trienal | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 19317-11-4 |
| Pubchem (cid): | 68150 |
| Pubchem (sid): | 135043794 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| KEGG (GenomeNet): | C03461 |
| HMDB (The Human Metabolome Database): | HMDB60356 |
| FooDB: | FDB014515 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
Potential Blenders and core components note
| For Odor | ||
| aldehydic | ||
| iso | butyraldehyde | FL/FR |
| floral | ||
| cyclohexyl propanol | FR | |
| earthy indane | FR | |
| floral methanol | FR | |
| ylang ylang flower oil CO2 extract | FL/FR | |
| ylang ylang flower oil I | FL/FR | |
| ylang ylang flower oil II | FL/FR | |
| ylang ylang flower oil III | FL/FR | |
| minty | ||
| dihydrocarveol | FL/FR | |
| For Flavor | ||
| aldehydic | ||
| aldehydic | ||
| iso | butyraldehyde | FL/FR |
| floral | ||
| ylang ylang flower oil CO2 extract | FL/FR | |
| ylang ylang flower oil I | FL/FR | |
| ylang ylang flower oil II | FL/FR | |
| ylang ylang flower oil III | FL/FR | |
| green | ||
| dihydrocarveol | FL/FR | |
Potential Uses:
| floral | FR | |
| jasmin | FR | |
| narcissus | FR | |
| neroli | FR | |
| rose | FR | |
| tuberose | FR |
Occurrence (nature, food, other): note
| ginger rhizome Search Trop Picture | |
| ginger rhizome oil Search Trop Picture | |
| lemongrass plant Search Trop Picture | |
| tomato fruit Search Trop Picture |
Synonyms:
| farnesal, pract. | |
| 3,7,11- | trimethyl dodeca-2,6,10-trienone |
| 3,7,11- | trimethyl-2,6,10-dodecatrienal |
| 3,7,11- | trimethyldodeca-2,6,10-trienal |


