Articles:
2-acetylthiophene
Notes:
Organoleptic which contributes to several aromas
| Flavor Demo Formulas | ||
| CAS Number: | 88-15-3 | 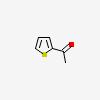 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 201-804-6 | |
| FDA UNII: | 5ASO208T20 | |
| Nikkaji Web: | J4.289G | |
| Beilstein Number: | 0107910 | |
| MDL: | MFCD00005442 | |
| CoE Number: | 11728 | |
| XlogP3: | 1.20 (est) | |
| Molecular Weight: | 126.17802000 | |
| Formula: | C6 H6 O S | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| DG SANTE Food Flavourings: | 15.040 2-acetylthiophene |
Physical Properties:
| Appearance: | colorless to pale yellow clear liquid (est) |
| Assay: | 98.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 1.16400 to 1.17100 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 9.686 to 9.744 |
| Refractive Index: | 1.56300 to 1.56900 @ 20.00 °C. |
| Melting Point: | 34.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 213.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.172000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 204.00 °F. TCC ( 95.56 °C. ) |
| logP (o/w): | 1.250 |
| Shelf Life: | 12.00 month(s) or longer if stored properly. |
| Storage: | store in cool, dry place in tightly sealed containers, protected from heat and light. |
| Soluble in: | |
| alcohol | |
| water, 14000 mg/L @ 30 °C (exp) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Type: sulfurous | |
| sulfurous nutty hazelnut walnut | |
| Odor Description: at 100.00 %. | sulfurous nutty hazelnut walnut |
| Flavor Type: onion | |
| onion malty roasted | |
| Taste Description: | onion, malty, roasted |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| T - Toxic. | |
|
R 25 - Toxic if swallowed. S 02 - Keep out of the reach of children. S 20/21 - When using do not eat, drink or smoke. S 23 - Do not breath vapour. S 36/37/39 - Wear suitable clothing, gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intraperitoneal-mouse LD50 40 mg/kg National Technical Information Service. Vol. AD691-490 | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 0.049 (μg/capita/day) | ||
| Modified Theoretical Added Maximum Daily Intake (mTAMDI): | 78 (μg/person/day) | ||
| Threshold of Concern: | 540 (μg/person/day) | ||
| Structure Class: | II | ||
| Food categories according to Commission Regulation EC No. 1565/2000 (EC, 2000) in FGE.06 (EFSA, 2002a). According to the Industry the "normal" use is defined as the average of reported usages and "maximum use" is defined as the 95th percentile of reported usages (EFSA, 2002i). | |||
| Note: mg/kg = 0.001/1000 = 0.000001 = 1/1000000 = ppm. | |||
| average usage mg/kg | maximum usage mg/kg | ||
| Dairy products, excluding products of category 02.0 (01.0): | 0.20000 | 1.00000 | |
| Fats and oils, and fat emulsions (type water-in-oil) (02.0): | 0.10000 | 0.50000 | |
| Edible ices, including sherbet and sorbet (03.0): | 0.20000 | 1.00000 | |
| Processed fruit (04.1): | 0.20000 | 1.00000 | |
| Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds (04.2): | - | - | |
| Confectionery (05.0): | 0.20000 | 1.00000 | |
| Chewing gum (05.0): | - | - | |
| Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery (06.0): | 0.10000 | 0.50000 | |
| Bakery wares (07.0): | 0.20000 | 1.00000 | |
| Meat and meat products, including poultry and game (08.0): | 0.10000 | 0.20000 | |
| Fish and fish products, including molluscs, crustaceans and echinoderms (MCE) (09.0): | 0.10000 | 0.20000 | |
| Eggs and egg products (10.0): | - | - | |
| Sweeteners, including honey (11.0): | - | - | |
| Salts, spices, soups, sauces, salads, protein products, etc. (12.0): | 0.10000 | 0.50000 | |
| Foodstuffs intended for particular nutritional uses (13.0): | 0.20000 | 1.00000 | |
| Non-alcoholic ("soft") beverages, excl. dairy products (14.1): | 0.10000 | 0.50000 | |
| Alcoholic beverages, incl. alcohol-free and low-alcoholic counterparts (14.2): | 0.40000 | 2.00000 | |
| Ready-to-eat savouries (15.0): | 0.40000 | 2.00000 | |
| Composite foods (e.g. casseroles, meat pies, mincemeat) - foods that could not be placed in categories 01.0 - 15.0 (16.0): | 0.10000 | 0.50000 | |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Flavouring Group Evaluation 21: Thiazoles, thiophene, thiazoline and thienyl derivatives from chemical group 29. Miscellaneous substances from chemical group 30. (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 76, (FGE.76)[1] - Consideration of sulphur-containing heterocyclic compounds evaluated by JECFA (59th meeting) structurally related to thiazoles, thiophene, thiazoline and thienyl derivatives from chemical group 29, miscellaneous substances from chemical group 30 evaluated by EFSA in FGE.21 - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food View page or View pdf | |
| Flavouring Group Evaluation 21, Revision 1 (FGE.21Rev1): Thiazoles, thiophene, thiazoline and thienyl derivatives from chemical group 29 Miscellaneous substances from chemical group 30 View page or View pdf | |
| Flavouring Group Evaluation 93 (FGE.93): Consideration of sulphur heterocyclic evaluated by JECFA (68th meeting) structurally related to thiazoles, thiophene, thiazoline and thienyl evaluated by EFSA in FGE.21Rev1 (2009) View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 21, Revision 2 (FGE.21Rev2): Thiazoles, thiophene, thiazoline and thienyl derivatives from chemical group 29. Miscellaneous substances from chemical group 30. View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 21, Revision 3 (FGE.21Rev3): Thiazoles, thiophenes, thiazoline and thienyl derivatives from chemical groups 29 and 30 View page or View pdf | |
| Statement on List of Representative Substances for Testing.
The current Statement lays down a list of substances in sub-groups with representative substances for which additional data are required prior to their evaluation through the Procedure (Regulation (EC) No 1565/2000). View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 21, Revision 4 (FGE.21Rev4): Thiazoles, thiophenes, thiazoline and thienyl derivatives from chemical groups 29 and 30 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 21, Revision 5 (FGE.21Rev5): Thiazoles, thiophenes, thiazoline and thienyl derivatives from chemical groups 29 and 30 View page or View pdf | |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 88-15-3 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 6920 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 2810 |
| WGK Germany: | 3 |
| 1-thiophen-2-ylethanone | |
| Chemidplus: | 0000088153 |
| RTECS: | OB6300000 for cas# 88-15-3 |
References:
| 1-thiophen-2-ylethanone | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 88-15-3 |
| Pubchem (cid): | 6920 |
| Pubchem (sid): | 134972021 |
| Flavornet: | 88-15-3 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEMBL: | View |
| HMDB (The Human Metabolome Database): | HMDB33133 |
| FooDB: | FDB011134 |
| YMDB (Yeast Metabolome Database): | YMDB01431 |
| Export Tariff Code: | 2934.99.9000 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
Potential Blenders and core components note
| For Odor | ||
| camphoreous | ||
| butyrophenone | FL/FR | |
| chocolate | ||
| 2- | methoxy-3-methyl pyrazine | FL/FR |
| nutty | ||
| 5- | acetyl-4-methyl thiazole | |
| 2- | methyl-3-pentenoic acid | FL/FR |
| For Flavor | ||
| No flavor group found for these | ||
| 5- | acetyl-4-methyl thiazole | |
| butyrophenone | FL/FR | |
| 2- | methyl-3-pentenoic acid | FL/FR |
| pyrazinyl methyl sulfide | FL | |
| alliaceous | ||
| 3- | mercapto-2-pentanone | FL |
| bitter | ||
| methyl ethoxypyrazine | FL | |
| malty | ||
| hordeum vulgare extract (cereal grass) | FL | |
| nutty | ||
| 2,5- | diethyl-3-methyl pyrazine | FL |
| 2- | methoxy-3-methyl pyrazine | FL/FR |
Potential Uses:
| beef roasted beef | FL | |
| bread | FL | |
| coffee | FR | |
| pork | FL | |
| vegetable | FL | |
| velvety |
Occurrence (nature, food, other): note
| asparagus - trace amount Search Trop Picture | |
| beef Search PMC Picture | |
| bread Search PMC Picture | |
| coffee - up to 1.25 mg/kg Search PMC Picture | |
| kohlrabi - 0.02 mg/kg Search Trop Picture | |
| kohlrabi stem Search Trop Picture | |
| pork grilled, roasted pork - up to 0.00002 mg/kg Search PMC Picture | |
| vegetables Search Picture |
Synonyms:
| 2- | aceto thiophene |
| 2- | acetothienone |
| 2- | acetothiophene |
| alpha- | acetyl thiophene |
| 2- | acetylthiophene |
| ethanone, 1- (2-thienyl)- | |
| ethanone, 1-(2-thienyl)- | |
| ketone, methyl 2-thienyl | |
| methyl 2-thienyl ketone | |
| methyl-2-thienyl ketone | |
| 2- | thienyl methyl ketone |
| 1-(2- | thienyl) ethanone |
| 1-(2- | thienyl)-ethanone |
| 1-(2- | thienyl)ethan-1-one |
| 1-(2- | thienyl)ethanone |
| 1- | thiophen-2-yl-ethanone |
| 1-( | thiophen-2-yl)-ethanone |
| 1-( | thiophen-2-yl)ethan-1-one |
| 1-( | thiophen-2-yl)ethanone |
| 1- | thiophen-2-ylethanone |













