Articles:
2,6,6-trimethyl-1&2-cyclohexen-1-carboxaldehyde
Notes:
Constit. of saffron and many other plant materials. Prod. by Microcystis spp. A 50:50 mixt. with 2,6,6-Trimethyl-2-cyclohexene-1-carboxaldehyde JQM42-W is used as a flavouring ingredient
| Fragrance Demo Formulas | ||
| CAS Number: | 432-25-7 | 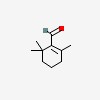 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 207-081-3 | |
| FDA UNII: | 77Y0U2X29G | |
| Nikkaji Web: | J127.248I | |
| MDL: | MFCD00079078 | |
| CoE Number: | 11849 | |
| XlogP3-AA: | 2.40 (est) | |
| Molecular Weight: | 152.23672000 | |
| Formula: | C10 H16 O | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 979 2,6,6-trimethyl-1&2-cyclohexen-1-carboxaldehyde |
| DG SANTE Food Flavourings: | 05.121 2,6,6-trimethyl-1-cyclohexen-1-carboxaldehyde |
| FEMA Number: | 3639 2,6,6-trimethyl-1&2-cyclohexen-1-carboxaldehyde |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 977045-71-8 ; 2,6,6-TRIMETHYL-1 AND 2-CYCLOHEXEN-1-CARBOXALDEHYDE |
Physical Properties:
| Appearance: | colorless to pale yellow clear liquid (est) |
| Assay: | 99.00 to 100.00 % sum of isomers |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.95000 to 0.95700 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 7.905 to 7.963 |
| Refractive Index: | 1.47600 to 1.48300 @ 20.00 °C. |
| Boiling Point: | 62.00 to 63.00 °C. @ 3.00 mm Hg |
| Boiling Point: | 211.00 to 212.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.176000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 170.00 °F. TCC ( 76.67 °C. ) |
| logP (o/w): | 3.100 (est) |
| Soluble in: | |
| alcohol | |
| water, 86.14 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor Type: tropical | |
| Odor Strength: | high , recommend smelling in a 10.00 % solution or less |
| Substantivity: | 85 hour(s) at 100.00 % |
| tropical saffron herbal clean rose sweet tobacco green fruity | |
| Odor Description: at 10.00 % in dipropylene glycol. | tropical saffron herbal clean rose oxide sweet tobacco damascone fruity Luebke, William tgsc, (2009) |
| Flavor Type: tropical | |
| tropical saffron herbal tobacco medicinal phenolic leathery green | |
| Taste Description: | tropical saffron herbal tobacco medicinal phenolic leathery green Luebke, William tgsc, (2009) |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents |
Suppliers:
| Augustus Oils |
| Beta Cyclocitral |
| Services |
| BOC Sciences |
| For experimental / research use only. |
| beta-Cyclocitral |
| Penta International |
| beta-CYCLOCITRAL |
| R C Treatt & Co Ltd |
| beta-Cyclocitral 95%
Halal, Kosher Odor: aromatic/camphor, berry/blackcurrant/raspberry, tea, and mango Flavor: tropical Used in chewing gum at 5ppm, frozen/hard confection at 1ppm, desserts and soft confection at 0.5ppm. |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| b-Cyclocitral |
| Sigma-Aldrich |
| b-Cyclocitral, ≥95%
Odor: fruity; green; minty. |
| Certified Food Grade Products |
| Synerzine |
| BETA-CYCLOCITRAL, (PURE) |
| Synerzine |
| beta-Cyclocitral, single isomer |
| Taytonn ASCC |
| beta-Cyclocitral |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 20/21/22 - Harmful by inhalation, in contact with skin and if swallowed. R 26/27/28 - Very toxic by inhalation, in contact with skin and if swallowed. S 02 - Keep out of the reach of children. S 20/21 - When using do not eat, drink or smoke. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 36/37/39 - Wear suitable clothing, gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for beta-cyclocitral usage levels up to: | |||
| 2.0000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 0.37 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | ND (μg/capita/day) | ||
| Structure Class: | I | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 12 | |||
| Click here to view publication 12 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | 0.06000 | |
| beverages(nonalcoholic): | - | 0.02000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | 0.50000 | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | 0.05000 | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | - | |
| fruit ices: | - | 0.10000 | |
| gelatins / puddings: | - | 0.05000 | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | 0.10000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | 0.05000 | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | 0.05000 | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of alicyclic substances used as flavor ingredients. View pdf | |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Scientific Opinion on Flavouring Group Evaluation 208 Revision 1 (FGE.208Rev1): Consideration of genotoxicity data on representatives for 10 alicyclic aldehydes with the a,�-unsaturation in ring / side-chain and precursors from chemical subgroup 2.2 of View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 208 Revision 2 (FGE.208Rev2): Consideration of genotoxicity data on alicyclic aldehydes with a,�-unsaturation in ring/side-chain and precursors from chemical subgroup 2.2 of FGE.19 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 208 Revision 3 (FGE.208Rev3): consideration of genotoxicity data on alicyclic aldehydes with a,�-unsaturation in ring/side-chain and precursors from chemical subgroup 2.2 of FGE.19 View page or View pdf | |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 432-25-7 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 9895 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| 2,6,6-trimethylcyclohexene-1-carbaldehyde | |
| Chemidplus: | 0000432257 |
References:
| 2,6,6-trimethylcyclohexene-1-carbaldehyde | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 432-25-7 |
| Pubchem (cid): | 9895 |
| Pubchem (sid): | 134974161 |
| Flavornet: | 432-25-7 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C20425 |
| HMDB (The Human Metabolome Database): | HMDB41011 |
| FooDB: | FDB020874 |
| Export Tariff Code: | 2912.19.0000 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
Potential Blenders and core components note
Potential Uses:
Occurrence (nature, food, other): note
| apricot fruit Search Trop Picture | |
| cassie absolute @ 0.37% Data GC Search Trop Picture | |
| champaca concrete @ trace% Data GC Search Trop Picture | |
| fig leaf Search Trop Picture | |
| gooseberry cape gooseberry fruit Search Trop Picture | |
| herniaria incana lam. oil greece @ 0.40% Data GC Search Trop Picture | |
| mango fruit Search Trop Picture | |
| oat seed Search Trop Picture | |
| plum fruit Search Trop Picture | |
| plumcot fruit Search PMC Picture | |
| safflower flower Search Trop Picture | |
| safflower leaf Search Trop Picture | |
| tea leaf Search Trop Picture | |
| tea shoot Search Trop Picture | |
| vanilla Search Picture | |
| water mint shoot Search Trop Picture |
Synonyms:
| .beta.- | cyclocitral |
| alpha & beta- | cyclocitral |
| b- | cyclocitral |
| beta- | cyclocitral |
| 1- | cyclohexene-1-carboxaldehyde, 2,6,6-trimethyl- |
| cyclohexenecarboxaldehyde, 2,6,6-trimethyl- | |
| 2,6,6- | trimethyl cyclohexene-1-carbaldehyde |
| 2,6,6- | trimethyl-1-cyclohexen-1-carboxaldehyde |
| 2,6,6- | trimethyl-1-cyclohexene-1-carbaldehyde |
| 2,6,6- | trimethyl-1-cyclohexene-1-carboxaldehyde |
| 2,6,6- | trimethyl-1(2)-cyclohexen-1-carboxaldehyde |
| 2,6,6- | trimethyl-1&2-cyclohexen-1-carboxaldehyde |
| 2,6,6- | trimethylcyclohex-1-ene-1-carbaldehyde |
| 2,6,6- | trimethylcyclohexene-1-carbaldehyde |
| 2,6,6- | trimethylcyclohexenecarbaldehyde |






