|
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | orange or orange pink clear liquid (est) |
| Assay: | 95.00 to 100.00 %
|
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 1.14100 to 1.15000 @ 25.00 °C.
|
| Pounds per Gallon - (est).: | 9.494 to 9.569
|
| Refractive Index: | 1.50900 to 1.53000 @ 20.00 °C.
|
| Boiling Point: | 57.00 to 60.00 °C. @ 44.00 mm Hg
|
| Vapor Pressure: | 5.782000 mmHg @ 25.00 °C. (est) |
| Vapor Density: | 3.9 ( Air = 1 ) |
| Flash Point: | 98.00 °F. TCC ( 36.67 °C. )
|
| logP (o/w): | 1.941 (est) |
| Shelf Life: | 6.00 month(s) or longer if stored properly. |
| Storage: | store in cool, dry place in tightly sealed containers, protected from heat and light. store under nitrogen. |
| Storage: | store under nitrogen. |
| Soluble in: |
| | alcohol | | | water, 630.8 mg/L @ 25 °C (est) |
| Insoluble in: |
| | water |
Organoleptic Properties:
| |
| Odor Type: sulfurous |
| |
| | sulfurous meaty fishy metallic |
Odor Description:
at 0.10 % in propylene glycol. | sulfury meaty fish metallic |
| |
| | sulfurous meaty fishy metallic chicken roasted chicken |
Odor Description:
| SuIfureous, meaty, fishy, metallic and roasted chicken-like
Mosciano, Gerard P&F 21, No. 3, 51, (1996) |
| |
| |
| Flavor Type: sulfurous |
| |
| | sulfurous fishy meaty salmon tuna roasted |
Taste Description:
at 15.00 ppm. | Sulfureous, fishy, meaty, salmon and tuna-like with a slight, roasted nuance
Mosciano, Gerard P&F 21, No. 3, 51, (1996) |
| |
| Odor and/or flavor descriptions from others (if found). |
| |
| Sigma-Aldrich |
| 2-Methyl-3-furanthiol, 95%, FG |
| Odor Description: | butter; coconut; coffee; meaty |
| Taste Description: | sulfury fish meat salmon tuna roasted |
| |
| R C Treatt & Co Ltd |
| 2-Methyl-3-furanthiol Halal, Kosher |
| Odor Description: | roasted meat; sweet beef broth, HVP notes |
| Taste Description: | roasted meat; sweet beef broth, HVP notes
Considered to be an important flavour chemical for meat flavours; also used in nut flavors. van den Ouweland, Demole & Enggist (1989) have noted that a 5- or 6- membered planar ring substituted with an "ene-thiol" and a methyl group adjacent to the thiol is important for the generation of meaty flavour/aroma. Normal use levels in finished consumer product: 0.005-0.25ppm. |
| |
| Taytonn ASCC |
| 2-Methyl-3-furanthiol |
| Odor Description: | Meaty, Roast, Powerful , Burnt |
| |
| Lluch Essence |
| 2-METHYL-3-FURANTHIOL 90% |
| Odor Description: | METALLIC, SULPHUROUS, ROASTED |
| |
| FCI SAS |
| 2-METHYL-3-MERCAPTO FURAN |
| Odor Description: | Roast meat, baked coffee |
| |
| |
Cosmetic Information:
Suppliers:
| Advanced Biotech |
| 2 METHYL 3 FURANTHIOL 1% ETHYL ACETATE NATURAL
|
| Advanced Biotech |
| 2 METHYL 3 FURANTHIOL 1% ETOH NATURAL
|
| Advanced Biotech |
| 2 METHYL 3 FURANTHIOL 1% IN SUNFLOWER OIL NATURAL
|
| Advanced Biotech |
| 2 METHYL 3 FURANTHIOL 1% OS NATURAL
|
| Advanced Biotech |
| 2 METHYL 3 FURANTHIOL 1% TRIACETIN NATURAL
|
| Advanced Biotech |
| 2 METHYL 3 FURANTHIOL 1% WS NATURAL
|
| Advanced Biotech |
| 2 METHYL 3 FURANTHIOL 5% ETHYL ACETATE NATURAL
|
| Advanced Biotech |
| 2 METHYL 3 FURANTHIOL 5% ETOH NATURAL
Odor: Alcoholic, Meaty, Roasted |
| Advanced Biotech |
| 2 METHYL 3 FURANTHIOL 5% TRIACETIN NATURAL
|
| Advanced Biotech |
| 2 METHYL 3-FURANTHIOL 5% SUNFLOWER OIL NATURAL
|
| Advanced Biotech |
| 2-METHYL-3-FURANTHIOL 5% OS NATURAL
Odor: Meaty, Roasted |
| Advanced Biotech |
| 2-METHYL-3-FURANTHIOL 5% WS NATURAL
5% min. (in WS) Odor: Meaty, Roasted |
| Advanced Biotech |
| 2-METHYL-3-FURANTHIOL
98% min. Odor: Meaty, Roasted |
| Ambles Nature et Chimie |
| 2 METHYL 3 FURANTHIOL NAT
|
| Anhui Suzhou Jinli Aromatic Chemicals |
| 2-Methyl-3-mercapto Furan
Odor: baked meat, fried coffee |
| Augustus Oils |
| 2 Methyl 3 Furanthiol
|
| Services |
| Aurochemicals |
| 2-2-METHYL-3-FURANTHIOL, Natural
|
| Aurochemicals |
| 2-METHYL-3-FURANTHIOL 1% IN ETOH, Natural
|
| Aurochemicals |
| 2-METHYL-3-FURANTHIOL 1% IN PG, Natural
|
| Aurochemicals |
| 2-METHYL-3-FURANTHIOL 5% IN PG, Natural
|
| Beijing Lys Chemicals |
| 2-Methyl-3-furanthiol
|
| BOC Sciences |
| For experimental / research use only. |
| 2-Methyl-3-mercapto-furan
|
| Charkit Chemical |
| METHYL-3-FURANTHIOL, 2- FEMA 3188
|
| Charkit Chemical |
| METHYL-3-FURANTHIOL, 2- NATURAL FEMA 3188
|
| DeLong Chemicals America |
| 2-Methyl-3-furanthiol, Kosher
|
| Endeavour Specialty Chemicals |
| 2-Methyl-3-furanthiol (0.1% in PG)
|
| Speciality Chemical Product Groups |
| Endeavour Specialty Chemicals |
| 2-Methyl-3-furanthiol (1% in PG)
|
| Endeavour Specialty Chemicals |
| 2-Methyl-3-furanthiol 98% F&F
|
| Ernesto Ventós |
| 2-METHYL-3-FURANTHIOL
|
| FCI SAS |
| 2-METHYL-3-MERCAPTO FURAN
Odor: Roast meat, baked coffee |
| Fleurchem |
| 2-methyl 3-furanthiol
|
| Frutarom |
| 2-METHYL-3-FURANTHIOL
KOSHER Flavor: Sulfurous, Meaty, Fishy, Metallic, Roasted, Burnt |
| CBD Offering |
| H. Interdonati, Inc. |
| 2-Methyl-3-mercaptofuran Kosher
|
| Featured Products |
| IFF |
| 2-METHYL-3-FURANTHIOL
KOSHER Flavor: Sulfurous, Meaty, Fishy, Metallic, Roasted, Burnt |
| Indukern F&F |
| 2-METHYL-3-FURANTHIOL
Odor: SULFUROUS, MEAT, FISH, METALIC |
| Jiangyin Healthway |
| 2-Methyl-3-mercapto furan (2-Methyl-3-furanthiol)
|
| New functional food ingredients |
| Jinan Enlighten Chemical Technology(Wutong Aroma ) |
| 2-Methyl-3-mercatpto furan, Kosherk
|
| Kingchem Laboratories |
| 2 METHYL-3-MERCAPTO FURAN (2-Methyl-3-furanthiol)
Odor: Roast meat |
| Lluch Essence |
| 2-METHYL-3-FURANTHIOL 90%
Odor: METALLIC, SULPHUROUS, ROASTED |
| M&U International |
| 2-METHYL-3-FURANTHIOL, Kosher
|
| Moellhausen |
| 2-METHYL-3-FURANTHIOL
|
| Natural Advantage |
| Methyl-3-Furanthiol, 2- Nat 5% in OH
Flavor: brothy, meaty, roasted, savory, sweet |
| Riverside Aromatics LTD.is the exclusive distributor for Europe in UK for any non-US based inquiries |
| Natural Advantage |
| Methyl-3-Furanthiol, 2- Nat 5% Triacetin Nat USOC
Flavor: brothy, meaty, roasted, savory, sweet |
| Natural Advantage |
| Methyl-3-Furanthiol, 2- Nat 5% Triacetin
Flavor: brothy, meaty, roasted, savory, sweet |
| OQEMA |
| 2-Methyl-3-Mercapto Furan
|
| Pearlchem Corporation |
| 2-Methyl-3-mercapto Furan
|
| Penta International |
| 2-METHYL-3-FURANTHIOL 5% IN NEOBEE
|
| Penta International |
| 2-METHYL-3-FURANTHIOL 0.1% IN Propylene Glycol
|
| Penta International |
| 2-METHYL-3-FURANTHIOL 1% IN PROPYLENE GLYCOL
|
| Penta International |
| 2-METHYL-3-FURANTHIOL 10% IN TRIACETIN
|
| Penta International |
| 2-METHYL-3-FURANTHIOL NATURAL 0.1% PROPYLENE GLYCOL
|
| Penta International |
| 2-METHYL-3-FURANTHIOL NATURAL 1% IN ETOH
|
| Penta International |
| 2-METHYL-3-FURANTHIOL NATURAL 1% IN NEOBEE
|
| Penta International |
| 2-METHYL-3-FURANTHIOL NATURAL 1% IN TRIACETIN
|
| Penta International |
| 2-METHYL-3-FURANTHIOL NATURAL 5% IN ETOH
|
| Penta International |
| 2-METHYL-3-FURANTHIOL NATURAL 5% IN SUNFLOWER OIL
|
| Penta International |
| 2-METHYL-3-FURANTHIOL NATURAL NEAT
|
| Penta International |
| 2-METHYL-3-FURANTHIOL SYNTHETIC 1% IN TRIACETIN
|
| Penta International |
| 2-METHYL-3-FURANTHIOL
|
| R C Treatt & Co Ltd |
| 2-Methyl-3-furanthiol, 0.1%
Halal, Kosher |
| R C Treatt & Co Ltd |
| 2-Methyl-3-furanthiol, 1% in PG
|
| R C Treatt & Co Ltd |
| 2-Methyl-3-furanthiol
Halal, Kosher Odor: roasted meat; sweet beef broth, HVP notes Flavor: roasted meat; sweet beef broth, HVP notes Considered to be an important flavour chemical for meat flavours; also used in nut flavors. van den Ouweland, Demole & Enggist (1989) have noted that a 5- or 6- membered planar ring substituted with an "ene-thiol" and a methyl group adjacent to the thiol is important for the generation of meaty flavour/aroma. Normal use levels in finished consumer product: 0.005-0.25ppm. |
| Riverside Aromatics |
| 2-METHYL-3-FURANTHIOL, NATURAL, 5% IN TRIACETIN
|
| Robinson Brothers |
| 2-Methyl-3-furanthiol (0.1% in PG)
|
| https://www.robinsonbrothers.uk/chemistry-competences |
| Robinson Brothers |
| 2-Methyl-3-furanthiol (1% in PG)
|
| Robinson Brothers |
| 2-Methyl-3-furanthiol F&F
|
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| 2-Methyl-3-furanthiol
|
| Sigma-Aldrich |
| 2-Methyl-3-furanthiol, 95%, FG
Odor: butter; coconut; coffee; meaty |
| Certified Food Grade Products |
| Sunaux International |
| 2-Methyl-3-furanthiol
|
| Synerzine |
| 2-Methyl-3-furanthiol
|
| Synerzine |
| Natural 2-Methyl-3-mercapto Furan
|
| Taytonn ASCC |
| 2-Methyl-3-furanthiol
Odor: Meaty, Roast, Powerful , Burnt |
| TCI AMERICA |
| For experimental / research use only. |
| 2-Methyl-3-furanthiol >95.0%(GC)
|
| Tengzhou Jitian Aroma Chemiclal |
| 2-Methyl-3-furanthiol
|
| Tengzhou Xiang Yuan Aroma Chemicals |
| 2-Methy-3-Mercapto Furan
|
| Tianjin Danjun International |
| 2-Methyl-3-furanthiol
|
| United International |
| 2-Methyl-3-mercapto furan;2-Methyl-3-Furanthiol
|
| WholeChem |
| 2-Methyl-3-mercapto furan (2-Methyl-
|
Safety Information:
| Preferred SDS: View |
| European information : |
| Most important hazard(s): | | T - Toxic. |
R 10 - Flammable.
R 25 - Toxic if swallowed.
S 01/02 - Keep locked up and out of the reach of children.
S 16 - Keep away from sources of ignition - No Smoking.
S 23 - Do not breath vapour.
S 24/25 - Avoid contact with skin and eyes.
S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S 36/37/39 - Wear suitable clothing, gloves and eye/face protection.
S 45 - In case of accident or if you feel unwell seek medical advice immediately.
|
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
| None found. |
| GHS Label elements, including precautionary statements |
| |
| Pictogram | |
| |
| Hazard statement(s) |
| None found. |
| Precautionary statement(s) |
| None found. |
| Oral/Parenteral Toxicity: |
gavage-mouse LD50 [sex: M,F] 100 mg/kg
(Oser, 1969a)
gavage-mouse LD50 [sex: M,F] 100 mg/kg
(Moran et al., 1980)
oral-mouse LD50 100 mg/kg
Drug and Chemical Toxicology. Vol. 3, Pg. 249, 1980.
|
| Dermal Toxicity: |
|
Not determined
|
| Inhalation Toxicity: |
|
Not determined
|
Safety in Use Information:
| Category: | flavor and fragrance agents |
| RIFM Fragrance Material Safety Assessment: Search |
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice |
| Recommendation for fish thiol usage levels up to: | | | 0.0100 % in the fragrance concentrate.
|
| |
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 0.52 (μg/capita/day) |
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 0.90 (μg/capita/day) |
| NOEL (No Observed Effect Level): | 5.00 (mg/kg bw per day) |
| Structure Class: | II |
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). |
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library |
| publication number: 4 |
| Click here to view publication 4 |
| | average usual ppm | average maximum ppm |
| baked goods: | - | 0.25000 |
| beverages(nonalcoholic): | - | - |
| beverages(alcoholic): | - | - |
| breakfast cereal: | - | - |
| cheese: | - | - |
| chewing gum: | - | - |
| condiments / relishes: | - | 0.25000 |
| confectionery froastings: | - | - |
| egg products: | - | - |
| fats / oils: | - | - |
| fish products: | - | - |
| frozen dairy: | - | - |
| fruit ices: | - | - |
| gelatins / puddings: | - | - |
| granulated sugar: | - | - |
| gravies: | - | 0.25000 |
| hard candy: | - | - |
| imitation dairy: | - | - |
| instant coffee / tea: | - | - |
| jams / jellies: | - | - |
| meat products: | - | 0.25000 |
| milk products: | - | - |
| nut products: | - | - |
| other grains: | - | - |
| poultry: | - | - |
| processed fruits: | - | - |
| processed vegetables: | - | - |
| reconstituted vegetables: | - | - |
| seasonings / flavors: | - | - |
| snack foods: | - | - |
| soft candy: | - | - |
| soups: | - | 0.25000 |
| sugar substitutes: | - | - |
| sweet sauces: | - | - |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): |
Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 13 (FGE.13); Furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14 (Commission Regulation (EC) No 1565/2000 of 18
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 13Rev1: Furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14
View page or View pdf |
Consideration of sulfur-substituted furan derivatives used as flavouring agents evaluated by JECFA (59th meeting) structurally related to a subgroup of substances within the group of �Furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14� evaluated by EFSA in FGE.13Rev1 (2009)
View page or View pdf |
Flavouring Group Evaluation 67 (FGE.67): Consideration of 40 furan-substituted aliphatic hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids and related esters, sulfides, disulfides and ethers evaluated by JECFA at the 65th meeting (JECFA, 2006b) and re-evaluated at the 69th meeting (JECFA, 2009c)
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 13, Revision 2 (FGE.13Rev2): Furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 67, Revision 1 (FGE.67Rev.1): Consideration of 40 furan-substituted aliphatic hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids and related esters, sulfides, disulfides and ethers evaluated by JECFA at the 65th meeting (JECFA, 2006b) and re-evaluated at the 69th meeting (JECFA, 2009c)
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 65, Revision 1 (FGE.65Rev1): Consideration of sulfur-substituted furan derivatives used as flavouring agents evaluated by JECFA (59th meeting) structurally related to a subgroup of substances within the group of �Furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14� evaluated by EFSA in FGE.13Rev2 (2011)
View page or View pdf |
Safety and efficacy of furfuryl and furan derivatives belonging to chemical group 14 when used as flavourings for all animal species and categories
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 13 Revision 3 (FGE.13Rev3): furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14
View page or View pdf |
| EPI System: | View |
| EPA Substance Registry Services (TSCA): | 28588-74-1 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 34286 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 1228 |
| WGK Germany: | 3 |
| | 2-methylfuran-3-thiol |
| Chemidplus: | 0028588741 |
| RTECS: | LU6235000 for cas# 28588-74-1 |
References:
Other Information:
Potential Blenders and core components note
| |
| For Odor |
| coffee |
| coffee |
| | coffee difuran | FL/FR |
| fishy |
| 1- | amino-2-propanol | CS |
| | triethyl amine | |
| floral |
| 6,8- | dimethyl-2-nonanol | FR |
| meaty |
| | meaty dithiane | FL/FR |
| 4- | methyl nonanoic acid | FL/FR |
| | ethyl 2-mercaptopropionate | FL/FR |
| 2- | mercaptopropionic acid | FL/FR |
| 4- | methyl 4-mercaptopentan-2-one 1% solution | FL/FR |
| |
| For Flavor |
| |
| No flavor group found for these |
| 2- | acetyl-1-pyrroline | FL |
| | amyl amine | FL |
| | benzyl methyl sulfide | FL |
| iso | butyl amine | FL |
| (±)-sec- | butyl amine | FL |
| | butyl amine | FL |
| | ethyl 4-(acetyl thio) butyrate | FL |
| | ethyl amine | FL |
| | hexyl amine | FL |
| 4- | methyl 4-mercaptopentan-2-one 1% solution | FL/FR |
| 2- | methyl butyl amine | FL |
| 2- | methyl-1-butane thiol | FL |
| 2- | methyl-3-furyl tetrasulfide | FL |
| 1,9- | nonane dithiol | FL |
| iso | pentylidene isopentyl amine | FL |
| | phenethyl amine | FL |
| 1,3- | propane dithiol | FL |
| | propionyl pyrroline 1% in vegatable oil triglyceride | FL |
| iso | propyl amine | FL |
| iso | propyl disulfide | FL |
| | triethyl amine | |
| (R)-N,N,alpha- | trimethyl benzyl amine | FL |
| | tripropyl amine | FL |
| alliaceous |
| 1,3- | butane dithiol | FL |
| 3- | mercapto-2-pentanone | FL |
| ammoniacal |
| 2- | methyl piperidine | FL |
| coffee |
| | coffee difuran | FL/FR |
| corn chip |
| 2- | acetyl-2-thiazoline | FL |
| earthy |
| | difurfuryl sulfide | FL |
| 1,8- | octane dithiol | FL |
| eggy |
| iso | propyl mercaptan | FL |
| fatty |
| 4- | methyl nonanoic acid | FL/FR |
| 2,4- | octadien-1-ol | FL |
| fishy |
| 4,5- | dimethyl thiazole | FL |
| 3- | penten-2-one | FL |
| | trimethyl amine | FL |
| green |
| 4- | penten-1-yl acetate | FL |
| meaty |
| 2,6- | dimethyl thiophenol | FL |
| 2,5- | dimethyl-3-furan thiol | FL |
| | furfuryl 2-methyl-3-furyl disulfide | FL |
| | meaty dithiane | FL/FR |
| 2- | mercaptopropionic acid | FL/FR |
| 2- | methyl 3-(methyl thio) furan | FL |
| S-(2- | methyl-3-furyl) ethane thioate | FL |
| 2- | methyl-3-tetrahydrofuran thiol | FL |
| | pyrazinyl ethane thiol | FL |
| ortho- | thiocresol | FL |
| metallic |
| 2,5- | dihydroxy-1,4-dithiane | FL |
| nutty |
| | nutty thiazole | FL |
| onion |
| | ethyl 2-mercaptopropionate | FL/FR |
| | furfuryl isopropyl sulfide | FL |
| roasted |
| | hexyl mercaptan | FL |
| sulfurous |
| 2,3- | butane dithiol | FL |
| | methyl 2-methyl-3-furyl disulfide | FL |
| | roasted butanol | FL |
| vegetable |
| | potato butyraldehyde | FL |
| |
Potential Uses:
Occurrence (nature, food, other): noteSynonyms:
| oxy | cyclothione (IFF) | | oxy | cyclothione-030 (IFF) | | | fish thiol | | | furan-3-thiol, 2-methyl- | | 3- | furanthiol, 2-methyl- | | 2- | methyl 3-furanthiol | | 2- | methyl furan-3-thiol | | 2- | methyl-3-furan thiol | | 2- | methyl-3-furanethiol | | 2- | methyl-3-furanthiol | | 2- | methyl-3-furanthiol 5% in ETOH | | 2- | methyl-3-furanthiol 5% in OS natural | | 2- | methyl-3-furanthiol 5% in sunflower oil natural | | 2- | methyl-3-furanthiol natural | | 2- | methyl-3-furanthiol, 0.1% in PG | | 2- | methyl-3-furyl mercaptan | | 2- | methyl-3-furylmercaptan | | 2- | methyl-3-furylthiol | | 2 | methyl-3-mercapto furan (2-methyl-3-furanthiol) | | 2- | methyl-3-mercapto-furan | | 2- | methyl-3-mercaptofuran | | 2- | methylfuran-3-thiol |
Articles:
| US Patents: | 5,470,991 - Process for the manufacture of furan derivatives |
| PubMed: | Relating sensory and chemical properties of sour cream to consumer acceptance. |
| PubMed: | Key aroma compounds in roasted in-shell peanuts. |
| PubMed: | Effect of addition of commercial rosemary extracts on potent odorants in cooked beef. |
| PubMed: | Metabolomic approach for determination of key volatile compounds related to beef flavor in glutathione-Maillard reaction products. |
| PubMed: | Synthesis and odor evaluation of five new sulfur-containing ester flavor compounds from 4-ethyloctanoic acid. |
| PubMed: | Selective preconcentration of volatile mercaptans in small SPE cartridges: quantitative determination of trace odor-active polyfunctional mercaptans in wine. |
| PubMed: | Identification of sulfur volatiles in canned orange juices lacking orange flavor. |
| PubMed: | Effect of cysteine and cystine addition on sensory profile and potent odorants of extruded potato snacks. |
| PubMed: | Key odor impact compounds in three yeast extract pastes. |
| PubMed: | Characterization of dried whey protein concentrate and isolate flavor. |
| PubMed: | Precursors of chicken flavor. II. Identification of key flavor precursors using sensory methods. |
| PubMed: | Characterization of the aroma of a meatlike process flavoring from soybean-based enzyme-hydrolyzed vegetable protein. |
| PubMed: | Effects of carnosine on volatile generation from Maillard reaction of ribose and cysteine. |
| PubMed: | 2-Methyl-3-furanthiol and methional are possible off-flavors in stored orange juice: aroma-similarity, NIF/SNIF GC-O, and GC analyses. |
| PubMed: | Quantitative gas chromatography-olfactometry carried out at different dilutions of an extract. Key differences in the odor profiles of four high-quality Spanish aged red wines. |
| PubMed: | Stability of thiols in an aqueous process flavoring. |
| PubMed: | Aroma extract dilution analysis of a beeflike process flavor from extruded enzyme-hydrolyzed soybean protein. |
| PubMed: | Structured fluids as microreactors for flavor formation by the Maillard reaction. |
| PubMed: | Environmental and industrial factors relating to flavor tainting of fish in the upper Wisconsin river. |
| PubMed: | Toxicological properties of thio- and alkylphenols causing flavor tainting in fish from the upper Wisconsin River. |
| PubMed: | A method for quantitative analysis of flavor-tainting alkylphenols and aromatic thiols in fish. |
| PubMed: | Identification of the volatile compounds produced in sterile fish muscle (Sebastes melanops) by Pseudomonas fragi. |
| PubMed: | Identification and origin of odorous sulfur compounds in cooked ham. |
| PubMed: | Comparison of aroma active and sulfur volatiles in three fragrant rice cultivars using GC-olfactometry and GC-PFPD. |
| PubMed: | Effects of additional cysteine in fish diet on mercury concentration. |
| PubMed: | Key aroma compounds in roasted in-shell peanuts. |
| PubMed: | Formation of cysteine-S-conjugates in the Maillard reaction of cysteine and xylose. |
| PubMed: | Effect of addition of commercial rosemary extracts on potent odorants in cooked beef. |
| PubMed: | Participation of cathepsin L in modori phenomenon in carp (Cyprinus carpio) surimi gel. |
| PubMed: | Oxidative changes during ice storage of rainbow trout (Oncorhynchus mykiss) fed different ratios of marine and vegetable feed ingredients. |
| PubMed: | Transglutaminase cross-linking effect on sensory characteristics and antioxidant activities of Maillard reaction products from soybean protein hydrolysates. |
| PubMed: | A new strategy for improved glutathione production from Saccharomyces cerevisiae: use of cysteine- and glycine-rich chicken feather protein hydrolysate as a new cheap substrate. |
| PubMed: | Development of a monoclonal antibody against the left wing of ciguatoxin CTX1B: thiol strategy and detection using a sandwich ELISA. |
| PubMed: | Reduction of mercury from mackerel fillet using combined solution of cysteine, EDTA, and sodium chloride. |
| PubMed: | Effects of thermal processing and various chemical substances on formaldehyde and dimethylamine formation in squid Dosidicus gigas. |
| PubMed: | Thermal denaturation of tilapia myosin and its subunits as affected by constantly increasing temperature. |
| PubMed: | Garlic-derived S-allylmercaptocysteine is a hepato-protective agent in non-alcoholic fatty liver disease in vivo animal model. |
| PubMed: | Visual detection of organophosphorus pesticides represented by mathamidophos using Au nanoparticles as colorimetric probe. |
| PubMed: | Metabolomic approach for determination of key volatile compounds related to beef flavor in glutathione-Maillard reaction products. |
| PubMed: | Assessment of the aroma impact of major odor-active thiols in pan-roasted white sesame seeds by calculation of odor activity values. |
| PubMed: | Effects of lard on the formation of volatiles from the Maillard reaction of cysteine with xylose. |
| PubMed: | Gelation properties of spent duck meat surimi-like material produced using acid-alkaline solubilization methods. |
| PubMed: | Recent patents on the use of antioxidant agents in food. |
| PubMed: | Comparative study on the stability of fish actomyosin and pork actomyosin. |
| PubMed: | The chemical nature of mercury in human brain following poisoning or environmental exposure. |
| PubMed: | Determination of dithiocarbamates and milneb residues in foods by gas chromatography-mass spectrometry. |
| PubMed: | Value-added use of mushroom ergothioneine as a colour stabilizer in processed fish meats. |
| PubMed: | Effect of various types of egg white on characteristics and gelation of fish myofibrillar proteins. |
| PubMed: | Methylmercury-induced neurotoxicity and apoptosis. |
| PubMed: | Comparative study of washing treatments and alkali extraction on gelation characteristics of striped catfish (Pangasius hypophthalmus) muscle protein. |
| PubMed: | The effect of arsenic exposure and the efficacy of DMSA on the proteins and lipids of the gill tissues of Labeo rohita. |
| PubMed: | Antioxidative activities of mushroom (Flammulina velutipes) extract added to bigeye tuna meat: dose-dependent efficacy and comparison with other biological antioxidants. |
| PubMed: | Quantification and odor contribution of 2-furanmethanethiol in different types of fermented soybean paste miso. |
| PubMed: | Off-flavors removal and storage improvement of mackerel viscera by supercritical carbon dioxide extraction. |
| PubMed: | Toxicant exposure and mental health--individual, social, and public health considerations. |
| PubMed: | Urine mercury excretion following meso-dimercaptosuccinic acid challenge in fish eaters. |
| PubMed: | Antioxidative activity and antidiscoloration efficacy of ergothioneine in mushroom (Flammulina velutipes) extract added to beef and fish meats. |
| PubMed: | Determination and isolation of a thioesterase from passion fruit (Passiflora edulis Sims) that hydrolyzes volatile thioesters. |
| PubMed: | Morphological changes due to Lead exposure and the influence of DMSA on the gill tissues of the freshwater fish, Catla catla. |
| PubMed: | Characterization of odor-active compounds in extracts obtained by simultaneous extraction/distillation from moroccan black olives. |
| PubMed: | Effect of cysteine and cystine addition on sensory profile and potent odorants of extruded potato snacks. |
| PubMed: | Homogenization conditions affect the oxidative stability of fish oil enriched milk emulsions: oxidation linked to changes in protein composition at the oil-water interface. |
| PubMed: | A new vapor generation system for mercury species based on the UV irradiation of mercaptoethanol used in the determination of total and methyl mercury in environmental and biological samples by atomic fluorescence spectrometry. |
| PubMed: | Effect of pH on the Maillard reaction of [13C5]xylose, cysteine, and thiamin. |
| PubMed: | Key odor impact compounds in three yeast extract pastes. |
| PubMed: | Aroma extraction dilution analysis of Sauternes wines. Key role of polyfunctional thiols. |
| PubMed: | Occurrence of polyfunctional thiols in fresh lager beers. |
| PubMed: | Beneficial effects of ascorbic acid on heat-induced fish gel (Kamaboko) from the superoxide anion radical. |
| PubMed: | A novel method for quantification of 2-methyl-3-furanthiol and 2-furanmethanethiol in wines made from Vitis vinifera grape varieties. |
| PubMed: | Precursors of chicken flavor. II. Identification of key flavor precursors using sensory methods. |
| PubMed: | Characterization of the aroma of a wine from maccabeo. Key role played by compounds with low odor activity values. |
| PubMed: | Efficacy of animal anti-fertility compounds against sea lamprey (Petromyzon marinus) spermatozoa. |
| PubMed: | Alpha-mercaptoketone formation during the maillard reaction of cysteine and [1-(13)C]ribose. |
| PubMed: | More on mercury content in fish. |
| PubMed: | More on mercury content in fish. |
| PubMed: | Cysteamine-a somatostatin-inhibiting agent-induced growth hormone secretion and growth acceleration in juvenile grass carp (Ctenopharyngodon idellus). |
| PubMed: | FT-Raman spectroscopic investigation of lens proteins of tilapia treated with dietary vitamin E. |
| PubMed: | Changes in conformation and subunit assembly of cod myosin at low and high pH and after subsequent refolding. |
| PubMed: | Studies on the metabolism of the thiofurans furfuryl mercaptan and 2-methyl-3-furanthiol in rat liver. |
| PubMed: | Odorants in breast milk. |
| PubMed: | The chemical form of mercury in fish. |
| PubMed: | Neurotoxicity of organomercurial compounds. |
| PubMed: | GC-olfactometric characterization of aroma volatiles from the thermal degradation of thiamin in model orange juice. |
| PubMed: | Formation of aroma compounds from ribose and cysteine during the Maillard reaction. |
| PubMed: | Gas chromatographic-olfactometric characterization of aroma compounds in two types of cashew apple nectar. |
| PubMed: | Mercury toxicity and antioxidants: Part 1: role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity. |
| PubMed: | Lipase-assisted generation of 2-methyl-3-furanthiol and 2-furfurylthiol from thioacetates. |
| PubMed: | Effects of carnosine on volatile generation from Maillard reaction of ribose and cysteine. |
| PubMed: | Characterization of the most odor-active compounds of Iberian ham headspace. |
| PubMed: | Chemical interactions between odor-active thiols and melanoidins involved in the aroma staling of coffee beverages. |
| PubMed: | Quantitative gas chromatography-olfactometry carried out at different dilutions of an extract. Key differences in the odor profiles of four high-quality Spanish aged red wines. |
| PubMed: | Stability of thiols in an aqueous process flavoring. |
| PubMed: | Partial purification of polyphenol oxidase from Chinese cabbage Brassica rapa L. |
| PubMed: | Aroma extract dilution analysis of a beeflike process flavor from extruded enzyme-hydrolyzed soybean protein. |
| PubMed: | Quantitative Model Studies on the Effectiveness of Different Precursor Systems in the Formation of the Intense Food Odorants 2-Furfurylthiol and 2-Methyl-3-furanthiol. |
| PubMed: | Structured fluids as microreactors for flavor formation by the Maillard reaction. |
| PubMed: | Olfactory discrimination of amino acids in brown bullhead catfish. |
| PubMed: | Production of monoclonal antibodies to Listeria monocytogenes and their application to determine the virulence of isolates from channel catfish. |
| PubMed: | Thietanium ion formation from the food mutagen 2-chloro-4-(methylthio)butanoic acid. |
| PubMed: | Purification and characterization of cathepsin L-like enzyme from the muscle of anchovy, Engraulis japonica. |
| PubMed: | Gastric carcinogenesis: 2-chloro-4-methylthiobutanoic acid, a novel mutagen in salted, pickled Sanma hiraki fish, or similarly treated methionine. |
| PubMed: | Increased susceptibility of liver to lipid peroxidation after ingestion of a high fish oil diet. |
| PubMed: | Excitotoxins in foods. |
| PubMed: | Interactive effects of selenium, methionine, and dietary protein on survival, growth, and physiology in mallard ducklings. |
| PubMed: | Interactive effects of arsenate, selenium, and dietary protein on survival, growth, and physiology in mallard ducklings. |
| PubMed: | Environmental and industrial factors relating to flavor tainting of fish in the upper Wisconsin river. |
| PubMed: | A method for quantitative analysis of flavor-tainting alkylphenols and aromatic thiols in fish. |
| PubMed: | Sensitive enzyme immunoassay of colistin and its application to detect residual colistin in rainbow trout tissue. |
| PubMed: | Effects of metals and organic compounds on hepatic glutathione, cysteine, and acid-soluble thiol levels in mullet (Mugil cephalus L.). |
| PubMed: | Effect of cysteine on iron absorption in man. |
| PubMed: | Protein changes in frozen fish. |
| PubMed: | Estimation of available methionine and cysteine in proteins of food products by in vivo and in vitro methods. |
| PubMed: | Mercury and mercurials. |
| PubMed: | Quantitative determination of the content of available methionine and cysteine in food proteins. |
| PubMed: | Identification of the volatile compounds produced in sterile fish muscle (Sebastes melanops) by Pseudomonas fragi. |
| PubMed: | Oxidation of methionine and cystine in foods treated with hydrogen peroxide. |
| PubMed: | Reduction of mercury with cysteine in comminuted halibut and hake fish protein concentrate. |
| PubMed: | Elimination of mercury from fish. |
| PubMed: | Volatile compounds produced in sterile fish muscle (Sebastes melanops) by Pseudomonas perolens. |
| PubMed: | Characterization of a major allergen (cod). Observations on effect of denaturation on the allergenic activity. |
| PubMed: | Symposium on microbial changes in foods. Bacteria active in the spoilage of certain sea foods. |
| PubMed: | Effect of amino acids on iron absorption from a staple vegetable food. |
| PubMed: | Determination of methylmercury salts in various kinds of biological material. |
|
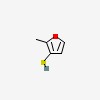 3D/inchi
3D/inchi





























