Articles:
methyl 2-pyrrolyl ketone
Notes:
Organoleptic which contributes to many aromas, including roasted filbert. Present in cooked apple, asparagus, wheat bread, tea, roasted peanut, popcorn, potato chips, licorice, Chinese boxthorn and other foodstuffs
| Fragrance Demo Formulas | ||
| CAS Number: | 1072-83-9 | 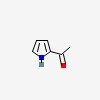 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 214-016-2 | |
| FDA UNII: | 9K28W7PM6N | |
| Nikkaji Web: | J80.142I | |
| Beilstein Number: | 0001882 | |
| MDL: | MFCD00005220 | |
| CoE Number: | 11721 | |
| XlogP3: | 0.90 (est) | |
| Molecular Weight: | 109.12799000 | |
| Formula: | C6 H7 N O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 1307 methyl 2-pyrrolyl ketone |
| DG SANTE Food Flavourings: | 14.047 2-acetylpyrrole |
| FEMA Number: | 3202 2-acetylpyrrole |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 1072-83-9 ; METHYL 2-PYRROLYL KETONE |
Physical Properties:
| Appearance: | beige to yellowish crystals (est) |
| Assay: | 97.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 87.00 to 93.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 220.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.110000 mmHg @ 25.00 °C. (est) |
| Flash Point: | > 212.00 °F. TCC ( > 100.00 °C. ) |
| logP (o/w): | 0.930 |
| Soluble in: | |
| alcohol | |
| water, 1.759e+004 mg/L @ 25 °C (est) | |
| water, 1.76E+04 mg/L @ C (exp) | |
| Stability: | |
| antiperspirant, fair | |
| concentrated fabric softener, fair | |
| hypochlorite bleach, fair | |
| liquid detergent, fair | |
| perborate powder detergent, good | |
| soap, good | |
Organoleptic Properties:
| Odor Type: musty | |
| musty nut skin cherry maraschino cherry coumarinic licorice bready walnut bready | |
| Odor Description: at 1.00 % in dipropylene glycol. | musty nut skin maraschino cherry coumarinic licorice walnut bready Luebke, William tgsc, (2017) |
| musty nutty coumarinic | |
| Odor Description: | Musty, nutty-like with a coumarin nuance Mosciano, Gerard P&F 16, No. 2, 49, (1991) |
| Flavor Type: nutty | |
| sweet fruity musty cherry nutty wasabi mustard tea | |
| Taste Description: | sweet fruity musty cherry nutty wasabi mustard tea Luebke, William tgsc, (2017) |
| sweet musty nutty tea | |
| Taste Description: at 10.00 ppm. | Sweet, musty, nutty and tea-like Mosciano, Gerard P&F 16, No. 2, 49, (1991) |
| Odor and/or flavor descriptions from others (if found). | |
| Takasago | |
| 2-Acetyl Pyrrole ≥98% as 2-Acetyl-1H-pyrrole | |
| Odor Description: | Walnut and licorice-like Used in flavors for tobacco and roasted foods. |
| Taste Description: | sweet musty nut tea |
| R C Treatt & Co Ltd | |
| 2-Acetylpyrrole Halal, Kosher | |
| Odor Description: | Bread, walnut, and important in liquorice |
| Taste Description: | nutty Used in flavours for beverages at 3ppm, and desserts at 2ppm, and in processed foods at 20-50ppm. |
| Moellhausen | |
| 2-ACETYL PYRROLE | |
| Odor Description: | nut, sweet, licorice |
| Taste Description: | sweet, tea |
Cosmetic Information:
| None found |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 22 - Harmful if swallowed. R 37/38 - Irritating to respiratory system and skin. S 02 - Keep out of the reach of children. S 22 - Do not breath dust. S 24 - Avoid contact with skin. S 36/37/39 - Wear suitable clothing, gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for 2-acetyl pyrrole usage levels up to: | |||
| 1.0000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 3.30 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 0.20 (μg/capita/day) | ||
| Structure Class: | II | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 4 | |||
| Click here to view publication 4 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | - | |
| beverages(nonalcoholic): | - | 50.00000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | 50.00000 | |
| fruit ices: | - | 50.00000 | |
| gelatins / puddings: | - | 50.00000 | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | 50.00000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | 50.00000 | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 24 (FGE.24): Pyridine, pyrrole, indole and quinoline derivatives from chemical group 28 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) View page or View pdf | |
| Pyridine, pyrrole, indole and quinoline derivatives from chemical group 28 Flavouring Group Evaluation 24, Revision 1 - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 77 (FGE77) [1] - Consideration of Pyridine, Pyrrole and Quinoline Derivatives evaluated by JECFA (63rd meeting) structurally related to Pyridine, Pyrrole, Indole and Quinoline Derivatives evaluated by EFSA in FGE.24Rev1 (2008) View page or View pdf | |
| Statement on List of Representative Substances for Testing.
The current Statement lays down a list of substances in sub-groups with representative substances for which additional data are required prior to their evaluation through the Procedure (Regulation (EC) No 1565/2000). View page or View pdf | |
| Scientific opinion on Flavouring Group Evaluation 24, Revision 2 (FGE.24Rev2): Pyridine, pyrrole, indole and quinoline derivatives from chemical group 28 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 77, Revision 2 (FGE.77Rev2): Consideration of Pyridine, Pyrrole and Quinoline Derivatives evaluated by JECFA (63rd meeting) structurally related to Pyridine, Pyrrole, Indole and Quinoline Derivatives evaluated by EFSA in FGE.24Rev2 (2013) View page or View pdf | |
| Safety and efficacy of pyridine and pyrrole derivatives belonging to chemical group 28 when used as flavourings for all animal species View page or View pdf | |
| Scientific opinion on flavouring group evaluation 77, revision 3 (FGE.77Rev3): consideration of pyridine, pyrrole and quinoline derivatives evaluated by JECFA (63rd meeting) structurally related to pyridine, pyrrole, indole and quinoline derivatives evaluated by EFSA in FGE.24Rev2 View page or View pdf | |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 1072-83-9 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 14079 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| 1-(1H-pyrrol-2-yl)ethanone | |
| Chemidplus: | 0001072839 |
| RTECS: | OB5970000 for cas# 1072-83-9 |
References:
| 1-(1H-pyrrol-2-yl)ethanone | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 1072-83-9 |
| Pubchem (cid): | 14079 |
| Pubchem (sid): | 134980104 |
| Flavornet: | 1072-83-9 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| HMDB (The Human Metabolome Database): | HMDB35882 |
| FooDB: | FDB014664 |
| Export Tariff Code: | 2933.99.9700 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
Potential Uses:
| bread | FL | |
| chocolate cocoa | FL | |
| coffee | FR | |
| licorice | FR | |
| malt | FR | |
| peanut | FR | |
| popcorn | FR | |
| sugar brown sugar | FL | |
| tea | FL | |
| tobacco | FR | |
| walnut | FL |
Occurrence (nature, food, other): note
| almond roasted almond Search Trop Picture | |
| asparagus Search Trop Picture | |
| beef cooked beef Search PMC Picture | |
| beef roasted beef Search PMC Picture | |
| chicken cooked chicken Search PMC Picture | |
| chicory root oil @ 0.096% Data GC Search Trop Picture | |
| cichorium intybus l. root extract @ 3.77% Data GC Search Trop Picture | |
| cocoa Search Trop Picture | |
| coconut Search Trop Picture | |
| coffee Search PMC Picture | |
| licorice Search PMC Picture | |
| malt Search PMC Picture | |
| mustard white mustard Search Trop Picture | |
| peanut Search Trop Picture | |
| popcorn Search PMC Picture | |
| pork cooked pork Search PMC Picture | |
| potato baked potato Search Trop Picture | |
| potato chip Search PMC Picture | |
| potato roasted potato Search Trop Picture | |
| sesame seed Search Trop Picture | |
| soybean Search Trop Picture | |
| tea leaf Search Trop Picture | |
| tea plant Search Trop Picture | |
| tobacco leaf Search Trop Picture | |
| whiskey malt whiskey Search Picture |
Synonyms:
| 2- | acetopyrrole |
| 2- | acetyl-1H-pyrrole |
| 2- | acetylpyrrole |
| 2 | acetylpyrrole (methyl-2-pyrrolyl ketone) |
| 2- | acetylpyrrole FCC |
| 2- | acetylpyrrole natural 5% in ethyl alcohol |
| ethanone, 1-(1H-pyrrol-2-yl)- | |
| methyl 2-pyrrolyl ketone | |
| methyl pyrrol-2-yl ketone | |
| pyrrol-2-yl methyl ketone | |
| 1-(1H- | pyrrol-2-yl) ethanone |
| 1-(1H- | pyrrol-2-yl)-ethanone |
| 1-(1H- | pyrrol-2-yl)ethan-1-one |
| 1-(1H- | pyrrol-2-yl)ethanone |
| pyrrole-beta-methyl ketone | |
| 2- | pyrrolyl ethanone |
| 2- | pyrrolyl methyl ketone |
| 1-(2- | pyrrolyl)-1-ethanone |
| 2- | pyrrolylmethyl ketone |






















