|
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | colorless to pale yellow clear liquid (est) |
| Assay: | 98.00 to 100.00 %
|
| Food Chemicals Codex Listed: | Yes |
| Specific Gravity: | 1.04700 to 1.05400 @ 25.00 °C.
|
| Pounds per Gallon - (est).: | 8.712 to 8.770
|
| Refractive Index: | 1.43100 to 1.43400 @ 20.00 °C.
|
| Melting Point: | -31.00 °C. @ 760.00 mm Hg
|
| Boiling Point: | 207.00 to 208.00 °C. @ 760.00 mm Hg
|
| Boiling Point: | 75.00 to 80.00 °C. @ 7.00 mm Hg
|
| Acid Value: | 1.00 max. KOH/g
|
| Vapor Pressure: | 0.235000 mmHg @ 25.00 °C. (est) |
| Vapor Density: | 3.45 ( Air = 1 ) |
| Flash Point: | 205.00 °F. TCC ( 96.11 °C. )
|
| logP (o/w): | -0.270 |
| Soluble in: |
| | alcohol | | | fixed oils | | | water | | | water, 9.381e+004 mg/L @ 25 °C (est) |
Organoleptic Properties:
| |
| Odor Type: herbal |
| |
| Odor Strength: | medium |
| |
| Substantivity: | 2 hour(s) at 100.00 % |
| |
| | herbal sweet warm tobacco cocoa woody |
Odor Description:
at 100.00 %. | herbal sweet warm tobacco cocoa woody
Luebke, William tgsc, (1986) |
| |
| |
| Flavor Type: tonka |
| |
| | tonka coumarinic tobacco cocoa chocolate dark chocolate coconut |
Taste Description:
| sweet tonka coumarinic tobacco cocoa dark chocolate coconut
Luebke, William tgsc, (1986) |
| |
| Odor and/or flavor descriptions from others (if found). |
| |
| Bedoukian Research |
| gamma-VALEROLACTONE 98.0% (sum of isomers) |
| Odor Description: | A diffusive sweet, hay and tobacco-like odor with a strawberry sweetness
Toiletry fragrances. |
| Taste Description: | sweet
Vanilla and tobacco flavors |
| |
| |
Cosmetic Information:
Suppliers:
| Achiewell |
| For experimental / research use only. |
| g-Valerolactone
|
| ACS International |
| Valerolactone gamma
Odor: Herbal sweet warm tobacco cocoa woody |
| Operational Capabilities |
| Advanced Biotech |
| GAMMA VALEROLACTONE NATURAL
99% min. Odor: Herbaceous |
| Advanced Biotech |
| GAMMA VALEROLACTONE SYNTHETIC
95% min. Odor: Herbaceous |
| Apple Flavor & Fragrance |
| gamma-Valerolactone
|
| Artiste |
| gamma-Valerolactone Natural
|
| Augustus Oils |
| Gamma Valerolactone
|
| Services |
| Aurochemicals |
| gamma-VALEROLACTONE, Natural
|
| Beijing Lys Chemicals |
| gamma-Valerolactone
|
| Berjé |
| gamma-Valeractone
|
| Media |
| BeYonde |
| Gamma-Valerolactone
|
| BOC Sciences |
| For experimental / research use only. |
| gamma-VALEROLACTONE FCC 98.0%
|
| Citrus and Allied Essences |
| gamma-Valerolactone FCC
Odor: soft sweet, tobacco-like |
| Market Report |
| CJ Latta & Associates |
| GAMMA VALEROLACTONE
|
| Creatingperfume.com |
| gamma-Valerolactone FCC
Odor: diffusive sweet, hay and tobacco-like |
| Diffusions Aromatiques |
| gamma-VALEROLACTONE
|
| Ernesto Ventós |
| VALEROLACTONE GAMMA
Odor: SWEET, FRUITY-FLORAL |
| Excellentia International |
| gamma-Valerolactone
|
| Indenta Group |
| gamma-Valerolactone
|
| Indukern F&F |
| GAMMA-VALEROLACTONE
Odor: FRUITY, MILK, BUTTER |
| Inoue Perfumery |
| gamma-VALEROLACTONE
|
| Jiangyin Healthway |
| gamma-Valerolactone Natural99%
|
| New functional food ingredients |
| Kingchem Laboratories |
| GAMMA VALEROLACTONE
|
| Kun Shan P&A |
| gamma-Valerolactone
|
| Kun Shan P&A |
| Natural gamma-Valerolactone
|
| Lluch Essence |
| GAMMA-VALEROLACTONE NATURAL
|
| Lluch Essence |
| GAMMA-VALEROLACTONE
|
| M&U International |
| gamma-Valerolactone, Kosher
|
| M&U International |
| Nat.gamma-Valerolactone, Kosher
|
| Moellhausen |
| Gamma-VEROLACTONE
|
| Odowell Co.,ltd |
| Gamma-Valerolactone
PURITY: 99%MIN. |
| OQEMA |
| gamma-Valerolactone natural
|
| OQEMA |
| gamma-Valerolactone natural
|
| Pearlchem Corporation |
| gamma-Valerolactone
|
| Penta International |
| GAMMA-VALEROLACTONE NATURAL
|
| Penta International |
| GAMMA-VALEROLACTONE
|
| Primechem |
| Gamma-Valerolactone
|
| Quimdis |
| Gamma Valerolactone SODA
|
| R C Treatt & Co Ltd |
| gamma-Valerolactone
|
| Reincke & Fichtner |
| gamma-Valerolactone
|
| Riverside Aromatics |
| gamma-VALEROLACTONE
|
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| gamma-Valerolactone
|
| Shanghai Vigen Fine Chemical |
| gamma-Valerolactone
|
| Sigma-Aldrich |
| g-Valerolactone, ≥99%, FCC, FG
Odor: anise; herbaceous |
| Certified Food Grade Products |
| Sigma-Aldrich |
| g-Valerolactone, natural, 95%, FG
Odor: warm; sweet; herbac |
| Soda Aromatic |
| gamma-Valerolactone
|
| Sunaux International |
| gamma-Valerolactone
|
| Sunaux International |
| nat.gamma-Valerolactone
|
| Synerzine |
| gamma-Valerolactone
|
| TCI AMERICA |
| For experimental / research use only. |
| gamma-Valerolactone >98.0%(GC)
|
| Tianjin Danjun International |
| Gamma valerolactone
|
| United International |
| Gamma Valerolactone Nat.
|
| United International |
| Gamma Valerolactone
|
| Vigon International |
| Valerolactone Gamma Natural
|
| Vigon International |
| Valerolactone Gamma
Odor: SOFT, SWEET, TOBACCO-LIKE |
| WEN International |
| GAMMA-VALEROLACTONE Natural
|
| WholeChem |
| Gamma valerolactone
|
Safety Information:
| Preferred SDS: View |
| European information : |
| Most important hazard(s): | | Xi - Irritant |
R 36/38 - Irritating to skin and eyes.
S 02 - Keep out of the reach of children.
S 24/25 - Avoid contact with skin and eyes.
S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S 37/39 - Wear suitable gloves and eye/face protection.
|
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
| None found. |
| GHS Label elements, including precautionary statements |
| |
| Pictogram | |
| |
| Hazard statement(s) |
| None found. |
| Precautionary statement(s) |
| None found. |
| Oral/Parenteral Toxicity: |
gavage-rat LD50 8800 mg/kg
(Deichmann et al., 1945)
oral-rat LD50 > 5000 mg/kg
(Moreno, 1978e)
gavage-rabbit LD50 2480 mg/kg
(Deichmann et al., 1945)
oral-rabbit LD50 2480 mg/kg
BEHAVIORAL: GENERAL ANESTHETIC
LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION
BEHAVIORAL: MUSCLE WEAKNESS
Journal of Industrial Hygiene and Toxicology. Vol. 27, Pg. 263, 1945.
oral-rat LD50 8800 mg/kg
BEHAVIORAL: GENERAL ANESTHETIC
LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION
BEHAVIORAL: MUSCLE WEAKNESS
Journal of Industrial Hygiene and Toxicology. Vol. 27, Pg. 263, 1945.
|
| Dermal Toxicity: |
skin-rabbit LD50 > 5000 mg/kg
Food and Chemical Toxicology. Vol. 20, Pg. 847, 1982.
|
| Inhalation Toxicity: |
|
Not determined
|
Safety in Use Information:
| Category: | flavor and fragrance agents |
| RIFM Fragrance Material Safety Assessment: Search |
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice |
| Recommendation for gamma-valerolactone usage levels up to: | | | 10.0000 % in the fragrance concentrate.
|
| |
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 120.00 (μg/capita/day) |
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). |
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library |
| publication number: 3 |
| Click here to view publication 3 |
| | average usual ppm | average maximum ppm |
| baked goods: | - | 50.00000 |
| beverages(nonalcoholic): | - | 4.00000 |
| beverages(alcoholic): | - | - |
| breakfast cereal: | - | - |
| cheese: | - | - |
| chewing gum: | - | - |
| condiments / relishes: | - | - |
| confectionery froastings: | - | - |
| egg products: | - | - |
| fats / oils: | - | - |
| fish products: | - | - |
| frozen dairy: | - | 20.00000 |
| fruit ices: | - | 20.00000 |
| gelatins / puddings: | - | - |
| granulated sugar: | - | - |
| gravies: | - | - |
| hard candy: | - | 50.00000 |
| imitation dairy: | - | - |
| instant coffee / tea: | - | - |
| jams / jellies: | - | - |
| meat products: | - | - |
| milk products: | - | - |
| nut products: | - | - |
| other grains: | - | - |
| poultry: | - | - |
| processed fruits: | - | - |
| processed vegetables: | - | - |
| reconstituted vegetables: | - | - |
| seasonings / flavors: | - | - |
| snack foods: | - | - |
| soft candy: | - | - |
| soups: | - | - |
| sugar substitutes: | - | - |
| sweet sauces: | - | - |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): |
| The FEMA GRAS assessment of lactones used as flavor ingredients. View pdf |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): |
Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission related to - Flavouring Group Evaluation 10: Aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones from chemical groups 9, 13 and 30 - (Commission Regulation (EC) No 1565/2000 of 18 July 2000)
View page or View pdf |
Flavouring Group Evaluation 10, Revision 1 (FGE10 Rev1)[1] - Aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones from chemical groups 9, 13 and 30 - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC)
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 10, Revision 2 (FGE.10Rev2): Aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones from chemical groups 9, 13 and 30
View page or View pdf |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 108-29-2 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 7921 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 2 |
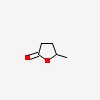
| | 5-methyloxolan-2-one |
| Chemidplus: | 0000108292 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | LU3580000 for cas# 108-29-2 |
References:
Other Information:
Potential Blenders and core components note
| |
| For Odor |
| aldehydic |
| aldehydic |
| 10- | undecenal (aldehyde C-11 undecylenic) | FL/FR |
| animal |
| | animal carbolactone | FR |
| | costus valerolactone | FR |
| balsamic |
| 2- | acetyl furan | FL/FR |
| | amyl cinnamate | FL/FR |
| iso | amyl cinnamate | FL/FR |
| | amyl phenyl acetate | FL/FR |
| | amyris wood oil | FL/FR |
| | benzyl cinnamate | FL/FR |
| dextro,laevo-iso | borneol | FL/FR |
| iso | bornyl acetate | FL/FR |
| | butyl cinnamate | FL/FR |
| | cinnamyl alcohol | FL/FR |
| | clover nitrile | FR |
| | ethyl cinnamate | FL/FR |
| | methyl cinnamate | FL/FR |
| | prenyl benzoate | FL/FR |
| berry |
| | raspberry ketone | FL/FR |
| buttery |
| | acetoin | FL/FR |
| camphoreous |
| dextro- | camphor | FL/FR |
| | cyclotene | FL/FR |
| | ethyl maltol | FL/FR |
| | fenugreek oleoresin | FL/FR |
| | maltol | FL/FR |
| | strawberry furanone | FL/FR |
| chocolate |
| iso | amyl phenyl acetate | FL/FR |
| iso | butyl phenyl acetate | FL/FR |
| | chocolate pyrazine A | FL/FR |
| | cocoa hexenal | FL/FR |
| | cocoa oleoresin | FL/FR |
| | cocoa pentenal | FL/FR |
| 2,5- | dimethyl pyrazine | FL/FR |
| 2,6- | dimethyl pyrazine | FL/FR |
| 2- | methoxypyrazine | FL/FR |
| 2,4,5- | trimethyl thiazole | FL/FR |
| citrus |
| blood | orange oil italy | FL/FR |
| cocoa |
| 2-iso | butyl-3,5-(and 3,6)-dimethyl pyrazine | FL/FR |
| 2- | methyl butyraldehyde | FL/FR |
| coconut |
| gamma- | heptalactone | FL/FR |
| gamma- | nonalactone (aldehyde C-18 (so-called)) | FL/FR |
| gamma- | octalactone | FL/FR |
| earthy |
| 2- | octanone | FL/FR |
| iso | propyl formate | FL/FR |
| fermented |
| 3- | methyl-1-pentanol | FL/FR |
| | valeraldehyde | FL/FR |
| floral |
| alpha- | amyl cinnamyl acetate | FL/FR |
| | amyl salicylate | FL/FR |
| iso | amyl salicylate | FL/FR |
| | anise indene | FR |
| | benzyl phenyl acetate | FL/FR |
| | coriander seed oil | FL/FR |
| | cyclamen aldehyde | FL/FR |
| | cyclohexyl ethyl alcohol | FL/FR |
| alpha- | damascone | FL/FR |
| | dihydrojasmone | FL/FR |
| | dimethyl anthranilate | FL/FR |
| | dimethyl benzyl carbinyl butyrate | FL/FR |
| | ethyl phenyl acetate | FL/FR |
| | floral pyranol | FR |
| | geraniol | FL/FR |
| | heliotropin | FL/FR |
| | heliotropyl acetate | FL/FR |
| | heliotropyl acetone | FL/FR |
| alpha- | hexyl cinnamaldehyde | FL/FR |
| | ilex paraguariensis oleoresin | FL/FR |
| (Z)- | jasmone | FL/FR |
| | karo karounde absolute | FR |
| | lavandula angustifolia flower oil | FL/FR |
| | lavender oil france | FL/FR |
| | leerall | FR |
| | linalool oxide | FL/FR |
| | methyl dihydrojasmonate | FL/FR |
| | nerolidol | FL/FR |
| | nonisyl propionate | FR |
| | ocean propanal | FL/FR |
| | orris rhizome resinoid (iris pallida) | FL/FR |
| | petitgrain bigarade oil | FL/FR |
| | prenyl salicylate | FL/FR |
| | rose butanoate | FL/FR |
| fruity |
| | allyl amyl glycolate | FR |
| | allyl cyclohexyl propionate | FL/FR |
| | artemisia pallens herb oil | FL/FR |
| 3- | benzyl-4-heptanone | FL/FR |
| | butyl 2-methyl butyrate | FL/FR |
| beta- | damascone | FL/FR |
| | ethyl levulinate | FL/FR |
| | green acetate | FR |
| | strawberry glycidate 1 (aldehyde C-16 (so-called)) | FL/FR |
| gamma- | undecalactone (aldehyde C-14 (so-called)) | FL/FR |
| green |
| iso | cyclocitral (IFF) | FL/FR |
| para- | methyl hydratropaldehyde | FL/FR |
| (E,Z)-2,6- | nonadien-1-ol | FL/FR |
| | phenyl acetaldehyde | FL/FR |
| | syringaldehyde | FL/FR |
| | valerian rhizome oil CO2 extract china | FL/FR |
| hay |
| | woodruff absolute | FR |
| herbal |
| | bornyl 2-methyl butyrate | FL/FR |
| | bornyl butyrate | FL/FR |
| beta- | bourbonene | FL/FR |
| alpha- | cadinol | FL/FR |
| | coriander oleoresin | FL/FR |
| | daucus carota fruit oil | FL/FR |
| | dimethyl cyclormol (IFF) | FR |
| | herbal undecanone | FR |
| | immortelle flower oil | FL/FR |
| | methyl nicotinate | FL/FR |
| curled | parsley leaf oil | FL/FR |
| | tea leaf absolute | FL/FR |
| | thyme oil wild or creeping | FL/FR |
| | tricyclodecenyl isobutyrate | FR |
| | tricyclodecenyl propionate | FR |
| | valerian rhizome oil | FL/FR |
| | valerian rhizome oil china | FL/FR |
| | yerba mate absolute | FL/FR |
| honey |
| | butyl phenyl acetate | FL/FR |
| | phenyl acetic acid | FL/FR |
| melon |
| | watermelon ketone | FR |
| minty |
| | pennyroyal oil | FL/FR |
| musty |
| | cocoa butenal | FL/FR |
| 3,6- | cocoa pyrazine | FL/FR |
| 4,5- | dimethyl-2-ethyl-3-thiazoline | FL/FR |
| 2,6- | lutidine | FL/FR |
| 2- | methyl pyrazine | FL/FR |
| 2,3,5,6- | tetramethyl pyrazine | FL/FR |
| 2,3,5- | trimethyl pyrazine | FL/FR |
| | vinyl sulfurol | FL/FR |
| phenolic |
| 2,3- | dimethyl benzofuran | FL/FR |
| popcorn |
| 2- | acetyl pyrazine | FL/FR |
| powdery |
| para- | anisyl alcohol | FL/FR |
| roasted |
| | fenugreek resinoid | FL/FR |
| spicy |
| | atractylis root oil | FR |
| | cassia bark oil china | FL/FR |
| | cinnamon acrolein | FL/FR |
| | clove bud oil | FL/FR |
| black | currant bud absolute | FL/FR |
| iso | eugenyl acetate | FL/FR |
| white | pepper oil | FL/FR |
| black | pepper oleoresin | FL/FR |
| 4-iso | propyl-2-cyclohexenone | FL/FR |
| | turmeric root absolute | FL/FR |
| terpenic |
| alpha- | terpineol | FL/FR |
| thujonic |
| common | tansy flower oil argentina | FL/FR |
| common | tansy leaf oil dutch | FR |
| | woody ketone | FL/FR |
| tobacco |
| 2,6,6- | trimethyl-2-hydroxycyclohexanone | FL/FR |
| tonka |
| | deertongue absolute | FR |
| gamma- | hexalactone | FL/FR |
| | tonka bean absolute | FR |
| vanilla |
| | ethyl vanillin | FL/FR |
| | ethyl vanillin isobutyrate | FL/FR |
| | vanilla bean absolute (vanilla planifolia) | FL/FR |
| | vanillin | FL/FR |
| | vanillyl acetate | FL/FR |
| | vanillyl isobutyrate | FL/FR |
| waxy |
| | phenethyl octanoate | FL/FR |
| woody |
| (R)-gamma- | cadinene | |
| | camphene | FL/FR |
| (+)- | camphene | FL/FR |
| | cistus twig/leaf oil | FL/FR |
| | marine formate | FR |
| gamma- | muurolene | |
| | origanum vulgare ssp. vulgare oil himalaya | |
| | polylimonene | FL/FR |
| | santall | FR |
| | spruce needle oil canada | FL/FR |
| (Z)- | woody amylene | FR |
| | woody cyclohexanone | FR |
| |
| For Flavor |
| |
| No flavor group found for these |
| | amyl phenyl acetate | FL/FR |
| | bornyl 2-methyl butyrate | FL/FR |
| | bornyl butyrate | FL/FR |
| beta- | bourbonene | FL/FR |
| | butyl cinnamate | FL/FR |
| 2-iso | butyl-3,5-(and 3,6)-dimethyl pyrazine | FL/FR |
| 2(4)-iso | butyl-4(2),6-dimethyl dihydro-4H-1,3,5-dithiazine | FL |
| delta- | cadinene | FL |
| (R)-gamma- | cadinene | |
| alpha- | cadinol | FL/FR |
| (+)- | camphene | FL/FR |
| | chocolate pyrazine A | FL/FR |
| | chocolate pyrazine B | FL |
| 4,5- | dimethyl-2-ethyl thiazole | FL |
| | fig leaf absolute | FL |
| | ilex paraguariensis oleoresin | FL/FR |
| 2- | methoxypyrazine | FL/FR |
| | methyl nicotinate | FL/FR |
| 1- | methyl pyrrole | FL |
| gamma- | muurolene | |
| | origanum vulgare ssp. vulgare oil himalaya | |
| | peanut dithiazine | FL |
| | polylimonene | FL/FR |
| | prenyl benzoate | FL/FR |
| 4-iso | propyl-2-cyclohexenone | FL/FR |
| 2,6,6- | trimethyl-2-hydroxycyclohexanone | FL/FR |
| | woody ketone | FL/FR |
|
| beta- | damascone | FL/FR |
| | paullinia cupana seed tincture | FL |
| apple |
| (E,Z)-2,6- | nonadien-1-ol | FL/FR |
| aromatic |
| | amyl salicylate | FL/FR |
| balsamic |
| | amyl cinnamate | FL/FR |
| | ethyl cinnamate | FL/FR |
| berry |
| | heliotropyl acetone | FL/FR |
| | raspberry ketone | FL/FR |
| bitter |
| | methyl ethoxypyrazine | FL |
| | paullinia cupana seed extract | FL |
| brown |
| | fenugreek oleoresin | FL/FR |
| camphoreous |
| dextro,laevo-iso | borneol | FL/FR |
| | camphene | FL/FR |
| caramellic |
| | caramel furanone | FL |
| | cyclotene | FL/FR |
| | ethyl maltol | FL/FR |
| | fenugreek resinoid | FL/FR |
| | maltol | FL/FR |
| | strawberry furanone | FL/FR |
| chemical |
| 2,3- | dimethyl benzofuran | FL/FR |
| cherry |
| | heliotropin | FL/FR |
| chocolate |
| | cocoa oleoresin | FL/FR |
| | cocoa propanal | FL |
| | creme de cocoa flavor | FL |
| citrus |
| blood | orange oil italy | FL/FR |
| | petitgrain bigarade oil | FL/FR |
| alpha- | terpineol | FL/FR |
| cocoa |
| iso | butyl phenyl acetate | FL/FR |
| | butyraldehyde | FL |
| | cocoa hexenal | FL/FR |
| 2- | methyl furan | FL |
| | syringaldehyde | FL/FR |
| coconut |
| gamma- | nonalactone (aldehyde C-18 (so-called)) | FL/FR |
| creamy |
| | acetoin | FL/FR |
| gamma- | hexalactone | FL/FR |
| gamma- | undecalactone (aldehyde C-14 (so-called)) | FL/FR |
| dairy |
| 2- | octanone | FL/FR |
| earthy |
| alpha- | amyl cinnamyl acetate | FL/FR |
| fatty |
| | cocoa butter distillates | FL |
| 10- | undecenal (aldehyde C-11 undecylenic) | FL/FR |
| floral |
| iso | amyl cinnamate | FL/FR |
| iso | amyl phenyl acetate | FL/FR |
| | cocoa pentenal | FL/FR |
| | dihydrojasmone | FL/FR |
| | dimethyl benzyl carbinyl butyrate | FL/FR |
| | geraniol | FL/FR |
| | heliotropyl acetate | FL/FR |
| | methyl dihydrojasmonate | FL/FR |
| | ocean propanal | FL/FR |
| | orris rhizome resinoid (iris pallida) | FL/FR |
| | phenyl acetic acid | FL/FR |
| | rosa canina seed extract | FL |
| fruity |
| | allyl cyclohexyl propionate | FL/FR |
| para- | anisyl alcohol | FL/FR |
| | artemisia pallens herb oil | FL/FR |
| 3- | benzyl-4-heptanone | FL/FR |
| | butyl 2-methyl butyrate | FL/FR |
| alpha- | damascone | FL/FR |
| | dimethyl anthranilate | FL/FR |
| | ethyl levulinate | FL/FR |
| | phenethyl octanoate | FL/FR |
| iso | propyl formate | FL/FR |
| | rose butanoate | FL/FR |
| | strawberry glycidate 1 (aldehyde C-16 (so-called)) | FL/FR |
| | valerian rhizome oil | FL/FR |
| | valerian rhizome oil china | FL/FR |
| | valerian rhizome oil CO2 extract china | FL/FR |
| fusel |
| 2- | methyl butyraldehyde | FL/FR |
| green |
| iso | amyl salicylate | FL/FR |
| | cinnamyl alcohol | FL/FR |
| | cocoa butenal | FL/FR |
| | cyclamen aldehyde | FL/FR |
| iso | cyclocitral (IFF) | FL/FR |
| | cyclohexyl ethyl alcohol | FL/FR |
| 2- | ethyl butyraldehyde | FL |
| | linalool oxide | FL/FR |
| para- | methyl hydratropaldehyde | FL/FR |
| | nerolidol | FL/FR |
| herbal |
| | coriander oleoresin | FL/FR |
| | coriander seed oil | FL/FR |
| | daucus carota fruit oil | FL/FR |
| | immortelle flower oil | FL/FR |
| | lavandula angustifolia flower oil | FL/FR |
| | lavender oil france | FL/FR |
| curled | parsley leaf oil | FL/FR |
| | prenyl salicylate | FL/FR |
| | thyme oil wild or creeping | FL/FR |
| | yerba mate absolute | FL/FR |
| honey |
| | benzyl phenyl acetate | FL/FR |
| | butyl phenyl acetate | FL/FR |
| | ethyl phenyl acetate | FL/FR |
| | phenyl acetaldehyde | FL/FR |
| lactonic |
| gamma- | heptalactone | FL/FR |
| gamma- | octalactone | FL/FR |
| malty |
| | yeast thiazoline | FL |
| meaty |
| 2,6- | dimethyl pyrazine | FL/FR |
| medicinal |
| dextro- | camphor | FL/FR |
| minty |
| | pennyroyal oil | FL/FR |
| musty |
| 2,5- | dimethyl pyrazine | FL/FR |
| | propionaldehyde | FL |
| 2,3,5- | trimethyl pyrazine | FL/FR |
| nutty |
| 2- | acetyl furan | FL/FR |
| 3,6- | cocoa pyrazine | FL/FR |
| 4,5- | dimethyl-2-ethyl-3-thiazoline | FL/FR |
| 2,4- | dimethyl-5-vinyl thiazole | FL |
| 2,6- | lutidine | FL/FR |
| 2- | methyl pyrazine | FL/FR |
| 2,3,5,6- | tetramethyl pyrazine | FL/FR |
| 2,4,5- | trimethyl thiazole | FL/FR |
| | vinyl sulfurol | FL/FR |
| roasted |
| 2- | acetyl pyrazine | FL/FR |
| rooty |
| | guarana flavor | FL |
| spicy |
| | benzyl cinnamate | FL/FR |
| | cassia bark oil china | FL/FR |
| | cinnamon acrolein | FL/FR |
| | clove bud oil | FL/FR |
| black | currant bud absolute | FL/FR |
| iso | eugenyl acetate | FL/FR |
| | methyl cinnamate | FL/FR |
| white | pepper oil | FL/FR |
| black | pepper oleoresin | FL/FR |
| | turmeric root absolute | FL/FR |
| tea |
| | tea leaf absolute | FL/FR |
| thujonic |
| common | tansy flower oil argentina | FL/FR |
| vanilla |
| | ethyl vanillin | FL/FR |
| | ethyl vanillin isobutyrate | FL/FR |
| | vanilla bean absolute (vanilla planifolia) | FL/FR |
| | vanillin | FL/FR |
| | vanillyl acetate | FL/FR |
| | vanillyl isobutyrate | FL/FR |
| waxy |
| alpha- | hexyl cinnamaldehyde | FL/FR |
| whiskey |
| 3- | methyl-1-pentanol | FL/FR |
| winey |
| | valeraldehyde | FL/FR |
| woody |
| | amyris wood oil | FL/FR |
| iso | bornyl acetate | FL/FR |
| | cistus twig/leaf oil | FL/FR |
| (Z)- | jasmone | FL/FR |
| | spruce needle oil canada | FL/FR |
| |
Potential Uses:
Occurrence (nature, food, other): noteSynonyms:
| | dihydro-5-methyl-2(3H)-furanone | | 4,5- | dihydro-5-methyl-2(3H)-furanone | | 2(3H)- | furanone, dihydro-5-methyl- | | 4- | hydroxypentanoic acid gamma-lactone | | 4- | hydroxyvaleric acid gamma-lactone | | 4- | hydroxyvaleric acid lactone | | 4- | methyl butan-4-olide | | 5- | methyl tetrahydro-2-furanone | | 4- | methyl-4-hydroxybutanoic acid lactone | | 4- | methyl-gamma-butyrolactone | | gamma- | methyl-gamma-butyrolactone | | 4- | methylbutan-4-olide | | 5- | methyldihydro-2(3H)-furanone | | 5- | methyldihydrofuran-2(3H)-one | | 5- | methyloxolan-2-one | | gamma- | pentalactone | | | pentano-1,4-lactone | | 4- | pentanolide | | gamma- | valeractone | | (±)-gamma- | valerolactone | | 4- | valerolactone | | gamma | valerolactone | | nat.gamma- | valerolactone | | gamma- | valerolactone FCC | | | valerolactone gamma | | | valerolactone gamma natural | | gamma- | valerolactone natural | | gamma- | valerolactone synthetic | | gamma- | valeryl lactone | | gamma- | verolactone |
Articles:
| PubMed: | Integration of mild acid hydrolysis in γ-valerolactone/water system for enhancement of enzymatic saccharification from cotton stalk. |
| PubMed: | Conversion of corn stalk into furfural using a novel heterogeneous strong acid catalyst in γ-valerolactone. |
| PubMed: | Atom-economical synthesis of γ-valerolactone with self-supplied hydrogen from methanol. |
| PubMed: | Effects of Metabolites Produced from (-)-Epigallocatechin Gallate by Rat Intestinal Bacteria on Angiotensin I-Converting Enzyme Activity and Blood Pressure in Spontaneously Hypertensive Rats. |
| PubMed: | Conversion of levulinic acid into γ-valerolactone using Fe3(CO)12: mimicking a biorefinery setting by exploiting crude liquors from biomass acid hydrolysis. |
| PubMed: | Porous Zirconium-Phytic Acid Hybrid: a Highly Efficient Catalyst for Meerwein-Ponndorf-Verley Reductions. |
| PubMed: | Insights into the Lactonase Mechanism of Serum Paraoxonase 1 (PON1): Experimental and Quantum Mechanics/Molecular Mechanics (QM/MM) Studies. |
| PubMed: | Acid-Functionalized Mesoporous Carbon: An Efficient Support for Ruthenium-Catalyzed γ-Valerolactone Production. |
| PubMed: | Profiling a gut microbiota-generated catechin metabolite's fate in human blood cells using a metabolomic approach. |
| PubMed: | Influence of age on the absorption, metabolism, and excretion of cocoa flavanols in healthy subjects. |
| PubMed: | Selective Hydrogenation of Furfural to Furfuryl Alcohol in the Presence of a Recyclable Cobalt/SBA-15 Catalyst. |
| PubMed: | In Situ Catalytic Hydrogenation of Biomass-Derived Methyl Levulinate to γ-Valerolactone in Methanol. |
| PubMed: | Cascade upgrading of γ-valerolactone to biofuels. |
| PubMed: | Solvent-enabled nonenyzmatic sugar production from biomass for chemical and biological upgrading. |
| PubMed: | High performing and stable supported nano-alloys for the catalytic hydrogenation of levulinic acid to γ-valerolactone. |
| PubMed: | Biotransformation of (-)-epigallocatechin and (-)-gallocatechin by intestinal bacteria involved in isoflavone metabolism. |
| PubMed: | A lignocellulosic ethanol strategy via nonenzymatic sugar production: process synthesis and analysis. |
| PubMed: | Titania-Supported Catalysts for Levulinic Acid Hydrogenation: Influence of Support and its Impact on γ-Valerolactone Yield. |
| PubMed: | Highly sensitive analysis of polyphenols and their metabolites in human blood cells using dispersive SPE extraction and LC-MS/MS. |
| PubMed: | Hydrodeoxygenation processes: advances on catalytic transformations of biomass-derived platform chemicals into hydrocarbon fuels. |
| PubMed: | Advanced biorefinery based on the fractionation of biomass in γ-valerolactone and water. |
| PubMed: | Solvent effects in acid-catalyzed biomass conversion reactions. |
| PubMed: | Efficient, solvent-free hydrogenation of α-angelica lactone catalysed by Ru/C at atmospheric pressure and room temperature. |
| PubMed: | Role of water in metal catalyst performance for ketone hydrogenation: a joint experimental and theoretical study on levulinic acid conversion into gamma-valerolactone. |
| PubMed: | Isolation and characterization of rat intestinal bacteria involved in biotransformation of (-)-epigallocatechin. |
| PubMed: | Catalytic conversion of γ-valerolactone to ε-caprolactam: towards nylon from renewable feedstock. |
| PubMed: | Is the age-related loss in olfactory sensitivity similar for light and heavy molecules? |
| PubMed: | Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. |
| PubMed: | Production of furfural from xylose, xylan and corncob in gamma-valerolactone using FeCl3·6H2O as catalyst. |
| PubMed: | Production of 4-valerolactone by an equilibrium-limited transformation in a partitioning bioreactor: impact of absorptive polymer properties. |
| PubMed: | Acet-oxy-γ-valerolactone. |
| PubMed: | Domino reaction catalyzed by zeolites with Brønsted and Lewis acid sites for the production of γ-valerolactone from furfural. |
| PubMed: | Conversion of carbohydrate biomass to γ-valerolactone by using water-soluble and reusable iridium complexes in acidic aqueous media. |
| PubMed: | Eco-solvents--cluster-formation, surfactantless microemulsions and facilitated hydrotropy. |
| PubMed: | Studies on the microbial synthesis and characterization of polyhydroxyalkanoates containing 4-hydroxyvalerate using γ-valerolactone. |
| PubMed: | RANEY® Ni catalyzed transfer hydrogenation of levulinate esters to γ-valerolactone at room temperature. |
| PubMed: | Facilitated uptake of a bioactive metabolite of maritime pine bark extract (pycnogenol) into human erythrocytes. |
| PubMed: | Uptake of gamma-valerolactone--detection of gamma-hydroxyvaleric acid in human urine samples. |
| PubMed: | Ionic-liquid-catalyzed efficient transformation of γ-valerolactone to methyl 3-pentenoate under mild conditions. |
| PubMed: | Electricity storage in biofuels: selective electrocatalytic reduction of levulinic acid to valeric acid or γ-valerolactone. |
| PubMed: | A chemo-enzymatic route to synthesize (S)-γ-valerolactone from levulinic acid. |
| PubMed: | Conversion of hemicellulose into furfural using solid acid catalysts in γ-valerolactone. |
| PubMed: | Electrophysiological responses of the olfactory receptors of the tick Amblyomma cajennense (Acari: Ixodidae) to host-related and tick pheromone-related synthetic compounds. |
| PubMed: | Development of heterogeneous catalysts for the conversion of levulinic acid to γ-valerolactone. |
| PubMed: | Structural elucidation and quantification of phenolic conjugates present in human urine after tea intake. |
| PubMed: | Origin of selectivity of Tsuji-Trost allylic alkylation of lactones: highly ordered transition states with lithium-containing enolates. |
| PubMed: | Facilitated cellular uptake and suppression of inducible nitric oxide synthase by a metabolite of maritime pine bark extract (Pycnogenol). |
| PubMed: | Production of aromatic hydrocarbons through catalytic pyrolysis of γ-valerolactone from biomass. |
| PubMed: | Inhibition of acetylcholinesterase by green and white tea and their simulated intestinal metabolites. |
| PubMed: | Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans. |
| PubMed: | Gamma butyrolactone (GBL) and gamma valerolactone (GVL): similarities and differences in their effects on the acoustic startle reflex and the conditioned enhancement of startle in the rat. |
| PubMed: | In vitro fermentation of a red wine extract by human gut microbiota: changes in microbial groups and formation of phenolic metabolites. |
| PubMed: | Conversion of biomass-derived levulinate and formate esters into γ-valerolactone over supported gold catalysts. |
| PubMed: | Liquid-phase catalytic transfer hydrogenation and cyclization of levulinic acid and its esters to γ-valerolactone over metal oxide catalysts. |
| PubMed: | Selective homogeneous hydrogenation of biogenic carboxylic acids with [Ru(TriPhos)H]+: a mechanistic study. |
| PubMed: | Hydrogen-independent reductive transformation of carbohydrate biomass into γ-valerolactone and pyrrolidone derivatives with supported gold catalysts. |
| PubMed: | Synthesis, analytical features, and biological relevance of 5-(3',4'-dihydroxyphenyl)-γ-valerolactone, a microbial metabolite derived from the catabolism of dietary flavan-3-ols. |
| PubMed: | Isolation of catechin-converting human intestinal bacteria. |
| PubMed: | Reactive extraction of levulinate esters and conversion to γ-valerolactone for production of liquid fuels. |
| PubMed: | Antioxidative activity of microbial metabolites of (-)-epigallocatechin gallate produced in rat intestines. |
| PubMed: | Simultaneous determination of γ-Hydroxybutyrate (GHB) and its analogues (GBL, 1.4-BD, GVL) in whole blood and urine by liquid chromatography coupled to tandem mass spectrometry. |
| PubMed: | Metabolism of green tea catechins by the human small intestine. |
| PubMed: | Conversion of levulinic acid and formic acid into γ-valerolactone over heterogeneous catalysts. |
| PubMed: | Plasma protein binding of polyphenols from maritime pine bark extract (USP). |
| PubMed: | Chemistry. Connecting biomass and petroleum processing with a chemical bridge. |
| PubMed: | γ-Valerolactone ring-opening and decarboxylation over SiO2/Al2O3 in the presence of water. |
| PubMed: | Integrated catalytic conversion of gamma-valerolactone to liquid alkenes for transportation fuels. |
| PubMed: | A methodology to estimate concentration profiles from two-dimensional covariance spectroscopy applied to kinetic data. |
| PubMed: | Bioavailability and catabolism of green tea flavan-3-ols in humans. |
| PubMed: | Palladium-catalyzed decarboxylative [4 + 3] cyclization of gamma-methylidene-delta-valerolactones with 1,1-dicyanocyclopropanes. |
| PubMed: | Profile of plasma and urine metabolites after the intake of almond [Prunus dulcis (Mill.) D.A. Webb] polyphenols in humans. |
| PubMed: | Characterization of two lactones in liquid phase: an experimental and computational approach. |
| PubMed: | Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography-tandem mass spectrometry. |
| PubMed: | Catalytic conversion of biomass-derived carbohydrates into gamma-valerolactone without using an external H2 supply. |
| PubMed: | MCR-ALS for sequential estimation of FTIR-ATR spectra to resolve a curing process using global phase angle convergence criterion. |
| PubMed: | Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-gamma-valerolactone as the major metabolites. |
| PubMed: | Human urinary metabolite profile of tea polyphenols analyzed by liquid chromatography/electrospray ionization tandem mass spectrometry with data-dependent acquisition. |
| PubMed: | Gamma-hydroxybutyrate concentrations in the blood of impaired drivers, users of illicit drugs, and medical examiner cases. |
| PubMed: | Maximising opportunities in supercritical chemistry: the continuous conversion of levulinic acid to gamma-valerolactone in CO(2). |
| PubMed: | Flavanol monomer-induced changes to the human faecal microflora. |
| PubMed: | Block copolymers for drug solubilisation: relative hydrophobicities of polyether and polyester micelle-core-forming blocks. |
| PubMed: | Towards 'bio-based' Nylon: conversion of gamma-valerolactone to methyl pentenoate under catalytic distillation conditions. |
| PubMed: | Cytoprotective constituent of Hoveniae Lignum on both Hep G2 cells and rat primary hepatocytes. |
| PubMed: | Single and multiple dose pharmacokinetics of maritime pine bark extract (pycnogenol) after oral administration to healthy volunteers. |
| PubMed: | "Ionic carbenes": synthesis, structural characterization, and reactivity of rare-Earth metal methylidene complexes. |
| PubMed: | Trends in gamma-hydroxybutyrate (GHB) and related drug intoxication: 1999 to 2003. |
| PubMed: | Analysis of GHB and 4-methyl-GHB in postmortem matrices after long-term storage. |
| PubMed: | A theoretical study on the origin of pi-facial stereoselectivity in the alkylation of enolates derived from 4-substituted gamma-butyrolactones. |
| PubMed: | Sequential arrangement of gamma-valerolactone enantiomers enclathrated in cholic acid channels as studied by 13C solid-state NMR: elucidation of the optical resolution mechanism. |
| PubMed: | Phenol and lactone receptors in the distal sensilla of the Haller's organ in Ixodes ricinus ticks and their possible role in host perception. |
| PubMed: | Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (pycnogenol). |
| PubMed: | Urinary excretion of 5-(3',4'-dihydroxyphenyl)-gamma-valerolactone, a ring-fission metabolite of (-)-epicatechin, in rats and its in vitro antioxidant activity. |
| PubMed: | Identification of metabolites of (-)-epicatechin gallate and their metabolic fate in the rat. |
| PubMed: | Clinical pharmacokinetics of antioxidants and their impact on systemic oxidative stress. |
| PubMed: | Pharmacokinetics of (-)-epicatechin-3-O-gallate, an active component of Onpi-to, in rats. |
| PubMed: | [Preparation of gas chromatographic capillary columns with beta-cyclodextrin polymer stationary phase modified with methyl phenyl silicone(OV-17)]. |
| PubMed: | Biosynthesis and local sequence specific degradation of poly(3-hydroxyvalerate-co-4-hydroxybutyrate) in Hydrogenophaga pseudoflava. |
| PubMed: | Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. |
| PubMed: | Urinary tea polyphenols in relation to gastric and esophageal cancers: a prospective study of men in Shanghai, China. |
| PubMed: | Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. |
| PubMed: | Urinary metabolites of French maritime pine bark extract in humans. |
| PubMed: | Metabolic fate of (-)-[4-(3)H]epigallocatechin gallate in rats after oral administration. |
| PubMed: | Versatile 8-oxabicyclo[3.2.1]oct-6-en-3-one: stereoselective methodology for generating C-glycosides, delta-valerolactones, and polyacetate segments. |
| PubMed: | Analysis of urinary metabolites of tea catechins by liquid chromatography/electrospray ionization mass spectrometry. |
| PubMed: | Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. |
| PubMed: | Pronounced differences in inhibition potency of lactone and non-lactone compounds for mouse and human coumarin 7-hydroxylases (CYP2A5 and CYP2A6). |
| PubMed: | Determination of free and glucuronated hexane metabolites without prior hydrolysis by liquid- and gas-chromatography coupled with mass spectrometry. |
| PubMed: | Comparison of thermal characteristics and degradation properties of epsilon-caprolactone copolymers. |
| PubMed: | Biosynthesis of poly(4-hydroxybutyric acid) by recombinant strains of Escherichia coli. |
| PubMed: | Method for the simultaneous quantification of n-hexane metabolites: application to n-hexane metabolism determination. |
| PubMed: | Toxic effects of hexane derivatives on cultured rat Schwann cells. |
| PubMed: | The metabolism of n-nonane in male Fischer 344 rats. |
| PubMed: | Identification of vertebrate volatiles stimulating olfactory receptors on tarsus I of the tick Amblyomma variegatum Fabricius (Ixodidae). I. Receptors within the Haller's organ capsule. |
| PubMed: | The influence of solvent stress on MMS-induced genetic change in Saccharomyces cerevisiae. |
| PubMed: | Induction of chromosome loss by mixtures of organic solvents including neurotoxins. |
| PubMed: | Aprotic polar solvents that affect porcine brain tubulin aggregation in vitro induce aneuploidy in yeast cells growing at low temperatures. |
| PubMed: | Urinary excretion of 2,5-hexanedione and peripheral polyneuropathies workers exposed to hexane. |
| PubMed: | Identification of the n-heptane metabolites in rat and human urine. |
| PubMed: | Impairment of human polymorphonuclear leukocyte chemotaxis by 2,5-hexanedione. |
| PubMed: | The microbial metabolism of condensed (+)-catechins by rat-caecal microflora. |
| PubMed: | Methodological investigations on the determination of n-hexane metabolites in urine. |
| PubMed: | Analysis of n-hexane, 2-hexanone, 2,5-hexanedione, and related chemicals by capillary gas chromatography and high-performance liquid chromatography. |
| PubMed: | Genetic change may be caused by interference with protein-protein interactions. |
| PubMed: | Identification of volatile metabolites of inhaled n-heptane in rat urine. |
| PubMed: | Changes of n-hexane neurotoxicity and its urinary metabolites by long-term co-exposure with MEK or toluene. |
| PubMed: | A study on biological monitoring of n-hexane exposure. |
| PubMed: | Changes of n-hexane metabolites in urine of rats exposed to various concentrations of n-hexane and to its mixture with toluene or MEK. |
| PubMed: | Urinary excretion of n-hexane metabolites. A comparative study in rat, rabbit and monkey. |
| PubMed: | Neurotoxic metabolites of "commercial hexane" in the urine of shoe factory workers. |
| PubMed: | Experimental neurotoxicity and urinary metabolites of the C5-C7 aliphatic hydrocarbons used as glue solvents in shoe manufacture. |
| PubMed: | Urinary excretion of the metabolites of n-hexane and its isomers during occupational exposure. |
| PubMed: | Measurement of the urinary metabolites of N-hexane, cyclohexane and their isomers by gas chromatography. |
| PubMed: | Pharmacokinetics of 14C-2-allophanyl-2-allyl -gamma-valero-lactone: a prodrug of proxibarbal in rats. |
| PubMed: | Cyclohexyl analogues of some antiinflammatory drugs. |
| PubMed: | Kinetics and mechanism of decomposition of some derivatives of 5-allylbarbituric acid. Part V. The course of hydrolysis of 5 allyl-5-(2'-hydroxypropyl)barbituric acid. |
| PubMed: | Central properties of alpha-allophanyl-alpha-allyl-gamma-valerolactone (valofan) [proceedings]. |
| PubMed: | Prevention of experimental gastric ulcers in rats by dial derivatives. |
| PubMed: | Presence of (+/-)-delta-(3,4-dihydroxyphenyl)-gamma-valerolactone in human urine. |
| PubMed: | Studies on flavonoid metabolism. Biliary and urinary excretion of metabolites of (+)-(U- 14 C)catechin. |
| PubMed: | New derivatives of alpha-ethyl-alpha-allophanyl-gamma-valerolactone. |
| PubMed: | Studies on flavonoid metabolism. Metabolism of (+)-[14C] catechin in the rat and guinea pig. |
| PubMed: | [Mutagenic action of alpha-aceto-beta-vinyl-gamma-valerolactone]. |
| PubMed: | 2-benzyl-5-(N,N-dimethylamino)-gamma-valerolactone, an inhibitor of plasma cholinesterase. |
| PubMed: | The influence of prolonged administration of alpha-allyl-allophanylphenyl-gamma-valerolactone (T1) on behavior and bioelectric brain activity in cats. |
| PubMed: | Studies on flavonoid metabolism. Metabolism of (+)-catechin in the guinea pig. |
| PubMed: | Metabolism of alpha-allyl-alpha-allophanyl-gamma-valerolactone. |
| PubMed: | DERIVATIVES OF ALPHA-PHENYL-ALPHA-ALLYL-GAMMA-VALEROLACTONE. |
| PubMed: | QUANTITATIVE DETERMINATION OF ALPHA-ALLYL-GAMMA-VALEROLACTONE IN PHYSIOLOGIC BODY FLUIDS. |
| PubMed: | Some derivatives of gamma-valerolactone. |
|
 3D/inchi
3D/inchi




























