Articles:
3,7-dihydro-1,3,7-trimethyl-1H-purine
Notes:
a methylxanthine naturally occurring in some beverages and also used as a pharmacological agent. caffeine's most notable pharmacological effect is as a central nervous system stimulant, increasing alertness and producing agitation. it also relaxes smooth muscle, stimulates cardiac muscle, stimulates diuresis, and appears to be useful in the treatment of some types of headache. several cellular actions of caffeine have been observed, but it is not entirely clear how each contributes to its pharmacological profile. among the most important are inhibition of cyclic nucleotide phosphodiesterases, antagonism of adenosine receptors, and modulation of intracellular calcium handling. Component of coffee beans (Coffea arabica), many other Coffea spp., chocolate (Theobroma cacao), tea (Camellia thea), kolanut (Cola acuminata) and several other Cola spp. and several other plants. Used in many cola-type beverages as a flavour enhancer
| CAS Number: | 58-08-2 | 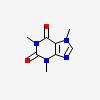 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 71701-02-5 | |
| ECHA EINECS - REACH Pre-Reg: | 200-362-1 | |
| FDA UNII: | 3G6A5W338E | |
| Nikkaji Web: | J2.330B | |
| Beilstein Number: | 0017705 | |
| MDL: | MFCD00005758 | |
| CoE Number: | 11741 | |
| XlogP3: | -0.10 (est) | |
| Molecular Weight: | 194.19410000 | |
| Formula: | C8 H10 N4 O2 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
Category: cosmetic and flavor agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| FDA/DG SANTE Petitions, Reviews, Notices: | |
| 182.1180 | Caffeine View - review |
| GRN 347 7 | Caffeine View - notice PDF |
| DG SANTE Food Flavourings: | 16.016 caffeine |
| FEMA Number: | 2224 caffeine |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 58-08-2 ; CAFFEINE |
| FDA Regulation: | |
| FDA PART 182 -- SUBSTANCES GENERALLY RECOGNIZED AS SAFE Subpart B--Multiple Purpose GRAS Food Substances Sec. 182.1180 Caffeine. | |
Physical Properties:
| Appearance: | white crystalline prisms (est) |
| Assay: | 98.00 to 100.00 % |
| Food Chemicals Codex Listed: | Yes |
| Melting Point: | 235.00 to 237.50 °C. @ 760.00 mm Hg |
| Boiling Point: | 416.79 °C. @ 760.00 mm Hg (est) |
| PH Number: | 6.50 |
| Vapor Pressure: | 15.000000 mmHg @ 89.00 °C. |
| Flash Point: | > 350.00 °F. TCC ( > 176.67 °C. ) |
| logP (o/w): | -0.070 |
| Shelf Life: | 24.00 month(s) or longer if stored properly. |
| Storage: | store in cool, dry place in tightly sealed containers, protected from heat and light. |
| Storage: | refrigerate in tightly sealed containers. |
| Soluble in: | |
| acetone, 1 gram in 50 ml acetone | |
| alcohol, 1 gram in 22 ml alcohol of 60% | |
| water, 1 gram in 46 ml water | |
| water, 45.5 grams in 100 ml water @ 65C | |
| water, 2.16E+04 mg/L @ 25 °C (exp) | |
Organoleptic Properties:
| Odor Type: odorless | |
| Odor Strength: | none |
| Odor Description: at 100.00 %. | odorless |
| Flavor Type: astringent | |
| astringent | |
| Taste Description: | astringent |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
fragrance skin conditioning |
Suppliers:
| AIDP |
| Caffeine (Natural) 98.5% min |
| AIDP |
| Caffeine 98.5%-101.5% |
| Allan Chemical |
| Caffeine |
| American International Chemical, LLC. |
| Caffeine |
| Atlantic Chemicals |
| Caffeine Anhydrous |
| AuNutra® Industries |
| Caffeine Anhydrous |
| Balchem |
| Caffeine
VitaShure® Flavor: characteristic Vitashure is an extensive line of microencapsulated vitamins, minerals, amino acids, and bioactive substances, specifically designed for the supplementation and fortification industries. |
| BASF |
| Caffeine Powder |
| Bell Flavors & Fragrances |
| Caffeine (USP Fine) |
| BOC Sciences |
| For experimental / research use only. |
| Caffeine min. 99%. (TLC) |
| Changsha Organic Herb |
| Guarana Seed Extract |
| Charkit Chemical |
| CAFFEINE ANHYDROUS USP SYNTHETIC 100 MESH NLG 85% |
| Charkit Chemical |
| CAFFEINE ANHYDROUS USP |
| Charkit Chemical |
| CAFFEINE, ANHYDROUS, USP SYNTHETIC |
| Coompo |
| For experimental / research use only. |
| Caffeine from Plants ≥95%
Odor: characteristic Use: Caffeine has many effects on the body's metabolism, including stimulating the central nervous system. This can make you more alert and give you a boost of energy.
For most people, the amount of caffeine in two to four cups of coffee a day is not harmful. However, too much caffeine can make you restless, anxious, and irritable. It may also keep you from sleeping well and cause headaches, abnormal heart rhythms, or other problems. If you stop using caffeine, you could get withdrawal symptoms.
Inside the body caffeine acts through several mechanisms, but its most important effect is to counteract a substance called adenosine that naturally circulates at high levels throughout the body, and especially in the nervous system. In the brain, adenosine plays a generally protective role, part of which is to reduce neural activity levels – for example, there is some evidence that adenosine helps to induce torpor in animals that seasonally hibernate.
Caffeine is toxic at sufficiently high doses. Ordinary consumption can have low health risks, even when carried on for years – there may be a modest protective effect against some diseases, including certain types of cancer. Caffeine can have both positive and negative effects on anxiety disorders. Some people experience sleep disruption if they consume caffeine, especially during the evening hours, but others show little disturbance and the effect of caffeine on sleep is highly variable.
Evidence of a risk to pregnancy is equivocal, but some authorities have concluded that prudent advice is for pregnant women to limit consumption to the equivalent of two cups of coffee per day or less. |
| ECSA Chemicals |
| CAFFEINE ANHYDROUS BP/USP |
| ECSA TRADE THE MOST UPDATED FINANCIAL PUBLICATION ON THE WORLD OF CHEMISTRY |
| Ernesto Ventós |
| NATURAL CAFFEINE ANHYDROUS |
| Foodchem International |
| Caffeine Anhydrous |
| George Uhe Company |
| Caffeine |
| Glentham Life Sciences |
| Caffeine |
| Graham Chemical |
| Caffeine |
| Kraft Chemical |
| Caffeine |
| M&U International |
| Caffeine, Kosher |
| Maypro Industries |
| Caffeine Anhydrous USP |
| Noble Molecular Research |
| For experimental / research use only. |
| Caffeine |
| Northwestern Extract |
| Caffeine Artificial or Natural |
| OQEMA |
| Natural Caffeine |
| Penta International |
| CAFFEINE POWDER ANHYDROUS FCC |
| Penta International |
| CAFFEINE POWDER ANHYDROUS USP FCC (SYNTHETIC) |
| Penta International |
| CAFFEINE POWDER NATURAL |
| Prinova |
| Caffeine |
| Sigma-Aldrich |
| Caffeine, anhydrous, FCC, 99% |
| Certified Food Grade Products |
| Silver Fern Chemical |
| Caffeine Anhydrous |
| Synthite Industries |
| Caffeine |
| E-books and Brochures |
| TCI AMERICA |
| For experimental / research use only. |
| Caffeine >98.0%(LC)(T) |
| Tianjin Talent Chemical |
| Caffeine |
| Vigon International |
| CAFFEINE NATURAL |
| WEN International |
| CAFFEINE Natural |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 22 - Harmful if swallowed. R 36/37/38 - Irritating to eyes, respiratory system, and skin. S 02 - Keep out of the reach of children. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 36/37/39 - Wear suitable clothing, gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 192 mg/kg KIDNEY, URETER, AND BLADDER: INTERSTITIAL NEPHRITIS BEHAVIORAL: WITHDRAWAL BRAIN AND COVERINGS: OTHER DEGENERATIVE CHANGES Journal of New Drugs. Vol. 5, Pg. 252, 1965. intravenous-rat LD50 105 mg/kg KIDNEY, URETER, AND BLADDER: STRUCTURAL OR FUNCTIONAL CHANGES IN URETER LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA Journal of Pharmacology and Experimental Therapeutics. Vol. 82, Pg. 89, 1944. intraperitoneal-rat LD50 240 mg/kg Zeitschrift fuer Ernaehrungswissenschaft. Vol. 15, Pg. 64, 1976. oral-rabbit LD50 224 mg/kg Zeitschrift fuer Ernaehrungswissenschaft. Vol. 15, Pg. 64, 1976. intravenous-rabbit LD50 58 mg/kg Kiso to Rinsho. Clinical Report. Vol. 13, Pg. 791, 1979. oral-mouse LD50 127 mg/kg GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" Toxicology and Applied Pharmacology. Vol. 44, Pg. 1, 1978. intravenous-mouse LD50 62 mg/kg LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD Toxicology Letters. Vol. 29, Pg. 25, 1985. intraperitoneal-mouse LD50 168 mg/kg KIDNEY, URETER, AND BLADDER: URINE VOLUME INCREASED BEHAVIORAL: AGGRESSION BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) Chemical and Pharmaceutical Bulletin. Vol. 22, Pg. 1459, 1974. oral-hamster LD50 230 mg/kg GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" Toxicology and Applied Pharmacology. Vol. 44, Pg. 1, 1978. oral-guinea pig LD50 230 mg/kg "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1335, 1935. oral-bird - wild LD50 316 mg/kg Archives of Environmental Contamination and Toxicology. Vol. 12, Pg. 355, 1983. intraperitoneal-cat LDLo 180 mg/kg "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1335, 1935. intravenous-cat LDLo 80 mg/kg "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1335, 1935. oral-cat LDLo 100 mg/kg "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1335, 1935. oral-child LDLo 320 mg/kg BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: CYANOSIS Forensic Science. Vol. 3, Pg. 275, 1974. oral-child TDLo 140 mg/kg BLOOD: HEMORRHAGE BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) CARDIAC: PULSE RATE INCREASE WITHOUT FALL IN BP Pediatric Emergency Care. Vol. 10, Pg. 349, 1994. oral-dog LD50 140 mg/kg Drugs in Japan Vol. 6, Pg. 174, 1982. intravenous-dog LDLo 4 mg/kg "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1335, 1935. parenteral-frog LDLo 120 mg/kg BEHAVIORAL: STIFFNESS BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD CARDIAC: OTHER CHANGES Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 166, Pg. 437, 1932. oral-guinea pig LD50 230 mg/kg BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION Toxicology and Applied Pharmacology. Vol. 2, Pg. 23, 1960. intraperitoneal-guinea pig LDLo 220 mg/kg "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1335, 1935. oral-human LDLo 192 mg/kg Journal of New Drugs. Vol. 5, Pg. 252, 1965. intravenous-human TDLo 7 mg/kg BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD Acta Pharmacologica et Toxicologica. Vol. 15, Pg. 331, 1959. oral-man TDLo 13 mg/kg BEHAVIORAL: TOXIC PSYCHOSIS American Journal of Psychiatry. Vol. 143, Pg. 1320, 1986. oral-man TDLo 51 mg/kg MUSCULOSKELETAL: TUMORS KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" CARDIAC: CHANGE IN RATE Annals of Emergency Medicine. Vol. 18, Pg. 94, 1989. unreported-mouse LD50 251 mg/kg Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol. 53, Pg. 2S, 1957. oral-rabbit LD50 224 mg/kg GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" Toxicology and Applied Pharmacology. Vol. 44, Pg. 1, 1978. intramuscular-rabbit LDLo 200 mg/kg Journal of Pharmacology and Experimental Therapeutics. Vol. 1, Pg. 572, 1910. intraperitoneal-rabbit LDLo 150 mg/kg "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1335, 1935. | |
| Dermal Toxicity: | |
|
subcutaneous-rat LD50 170 mg/kg Journal of Clinical Pharmacology and Journal of New Drugs. Vol. 7, Pg. 131, 1967. subcutaneous-mouse LD50 242 mg/kg Arzneimittel-Forschung. Drug Research. Vol. 6, Pg. 601, 1956. subcutaneous-cat LDLo 150 mg/kg "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1335, 1935. subcutaneous-dog LD50 100 mg/kg Drugs in Japan Vol. 6, Pg. 174, 1982. subcutaneous-frog LDLo 120 mg/kg Acta Pharmacologica et Toxicologica. Vol. 15, Pg. 331, 1959. subcutaneous-guinea pig LDLo 200 mg/kg "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1335, 1935. subcutaneous-rabbit LDLo 275 mg/kg Journal of Pharmacology and Experimental Therapeutics. Vol. 1, Pg. 572, 1910. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | cosmetic and flavor agents | ||
| Recommendation for caffeine usage levels up to: | |||
| not for fragrance use. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 380.00 (μg/capita/day) | ||
| Structure Class: | III | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 3. Update in publication number(s): 29 | |||
| Click here to view publication 3 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | - | |
| beverages(nonalcoholic): | 120.00000 | 150.00000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | - | |
| fruit ices: | - | - | |
| gelatins / puddings: | - | - | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | - | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
| Food categories according to Commission Regulation EC No. 1565/2000 (EC, 2000) in FGE.06 (EFSA, 2002a). According to the Industry the "normal" use is defined as the average of reported usages and "maximum use" is defined as the 95th percentile of reported usages (EFSA, 2002i). | |||
| Note: mg/kg = 0.001/1000 = 0.000001 = 1/1000000 = ppm. | |||
| average usage mg/kg | maximum usage mg/kg | ||
| Dairy products, excluding products of category 02.0 (01.0): | 20.00000 | 70.00000 | |
| Fats and oils, and fat emulsions (type water-in-oil) (02.0): | - | - | |
| Edible ices, including sherbet and sorbet (03.0): | 20.00000 | 70.00000 | |
| Processed fruit (04.1): | - | - | |
| Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds (04.2): | - | - | |
| Confectionery (05.0): | 25.00000 | 100.00000 | |
| Chewing gum (05.0): | - | - | |
| Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery (06.0): | - | - | |
| Bakery wares (07.0): | - | - | |
| Meat and meat products, including poultry and game (08.0): | - | - | |
| Fish and fish products, including molluscs, crustaceans and echinoderms (MCE) (09.0): | - | - | |
| Eggs and egg products (10.0): | - | - | |
| Sweeteners, including honey (11.0): | - | - | |
| Salts, spices, soups, sauces, salads, protein products, etc. (12.0): | - | - | |
| Foodstuffs intended for particular nutritional uses (13.0): | - | - | |
| Non-alcoholic ("soft") beverages, excl. dairy products (14.1): | 100.00000 | 175.00000 | |
| Alcoholic beverages, incl. alcohol-free and low-alcoholic counterparts (14.2): | 25.00000 | 100.00000 | |
| Ready-to-eat savouries (15.0): | - | - | |
| Composite foods (e.g. casseroles, meat pies, mincemeat) - foods that could not be placed in categories 01.0 - 15.0 (16.0): | - | - | |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Flavouring Group Evaluation 29 (FGE29)[1] - Substance from the priority list: Vinylbenzene from chemical group 31 - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 49, (FGE.49)[1]: Xanthin alkaloids from the Priority list from chemical group 30 View page or View pdf | |
| Gathering consumption data on specific consumer groups of energy drinks View page or View pdf | |
| Extensive literature search as preparatory work for the safety assessment for caffeine View page or View pdf | |
| Outcome of a public consultation on the draft Scientific Opinion of the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) on the safety of caffeine View page or View pdf | |
| EFSA explains risk assessment: Caffeine View page or View pdf | |
| Scientific Opinion on the safety of caffeine View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 49, Revision 1 (FGE.49Rev1): xanthine alkaloids from the priority list View page or View pdf | |
| Outcome of the consultation with Member States and EFSA on the basic substance application for approval of caffeine to be used in plant protection as insecticide in cabbage, potatoes and buxus and as molluscicide in all edible and non-edible crops View page or View pdf | |
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Daily Med: | search |
| NIOSH International Chemical Safety Cards: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| Carcinogenic Potency Database: | Search |
| EPA GENetic TOXicology: | Search |
| EPA Substance Registry Services (TSCA): | 58-08-2 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 2519 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 1544 |
| WGK Germany: | 1 |
| 1,3,7-trimethylpurine-2,6-dione | |
| Chemidplus: | 0000058082 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | 58-08-2 |
References:
| 1,3,7-trimethylpurine-2,6-dione | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 58-08-2 |
| Pubchem (cid): | 2519 |
| Pubchem (sid): | 134971358 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| Golm Metabolome Database: | Search |
| Metabolomics Database: | Search |
| UM BBD: | Search |
| KEGG (GenomeNet): | C07481 |
| HMDB (The Human Metabolome Database): | HMDB01847 |
| FooDB: | FDB002100 |
| Export Tariff Code: | 2939.30.0000 |
| FDA Listing of Food Additive Status: | View |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| Formulations/Preparations: •formulation names: caffedrine, vivarin, quick-pep •grades: technical; usp; fcc •nodoz keep alert tablets contain 100% caffeine in each tablet •vivarin contains 200 mg caffeine alkaloid and 150 mg dextrose in each tablet | |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| skin conditioning |
Occurrence (nature, food, other): note
| cacao bean Search Trop Picture | |
| cacao petiole Search Trop Picture | |
| cacao testa Search Trop Picture | |
| cherimoya seed Search Trop Picture | |
| clover yellow sweet clover Search Trop Picture | |
| coffee Search Trop Picture | |
| coffee arabica coffee Search Trop Picture | |
| coffee bean Search Trop Picture | |
| coffee canephora coffee Search Trop Picture | |
| coffee green coffee PbMd Search Trop Picture | |
| guarana Search Trop Picture | |
| kola Search Trop Picture | |
| lemon Search Trop Picture | |
| lemon flower Search Trop Picture | |
| orange bud Search Trop Picture | |
| orange flower Search Trop Picture | |
| orange leaf Search Trop Picture | |
| petitgrain orange Search Trop Picture | |
| pomelo Search Trop Picture | |
| tea anther Search Trop Picture | |
| tea flower Search Trop Picture | |
| tea green tea Search Trop Picture | |
| tea leaf Search Trop Picture | |
| tea pericarp Search Trop Picture | |
| tea plant Search Trop Picture | |
| tea seed Search Trop Picture | |
| tea seed coat Search Trop Picture | |
| tea shoot Search Trop Picture | |
| tea stem Search Trop Picture | |
| tea tissue culture Search Trop Picture |
Synonyms:
| biogenic caffeine-210 | |
| caffeine anhydrous | |
| caffeine anhydrous USP | |
| caffeine natural | |
| caffeine USP FCC powder anhydrous | |
| coffeine | |
| 3,7- | dihydro-1,3,7-trimethyl-1H-purine |
| guaranine | |
| methyl theobromine | |
| 7- | methyl theophylline |
| 1- | methyl-theobromine |
| 1H- | purine-2,6-dione, 3,7-dihydro-1,3,7-trimethyl- |
| theine | |
| 1,3,7- | trimethyl xanthine |
| 1,3,7- | trimethyl-1,3,7-trihydropurine-2,6-dione |
| 1,3,7- | trimethyl-1H-purine-2,6(3H,7H)-dione |
| 1,3,7- | trimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-dione |
| 1,3,7- | trimethyl-2,6-dioxo-1,2,3,6-tetrahydropurine |
| 1,3,7- | trimethyl-2,6-dioxopurine |
| 1,3,7- | trimethyl-3,7-dihydro-1H-purine-2,6-dione |
| 1,3,7- | trimethyl-3,7-dihydro-purine-2,6-dione |
| 1,3,7- | trimethylpurine-2,6-dione |











