|
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | colorless clear liquid (est) |
| Assay: | 92.00 to 100.00 % sum of isomers
|
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.97600 to 0.98600 @ 25.00 °C.
|
| Pounds per Gallon - (est).: | 8.121 to 8.205
|
| Refractive Index: | 1.42700 to 1.43500 @ 20.00 °C.
|
| Boiling Point: | 20.00 °C. @ 0.20 mm Hg
|
| Vapor Pressure: | 11.202000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 111.00 °F. TCC ( 43.89 °C. )
|
| logP (o/w): | 1.432 (est) |
| Soluble in: |
| | alcohol | | | water, slightly | | | water, 5261 mg/L @ 25 °C (est) |
Organoleptic Properties:
| |
| Odor Type: green |
| |
| Odor Strength: | high ,
recommend smelling in a 1.00 % solution or less |
| |
| Substantivity: | 240 hour(s) at 100.00 % |
| |
| | green fatty grassy weedy fruity apple |
Odor Description:
at 1.00 % in dipropylene glycol. | green fatty grassy weedy fruity apple
Luebke, William tgsc, (1990) |
| |
| |
| Flavor Type: green |
| |
| | sharp green grassy apple cooked apple apple skin |
Taste Description:
| sharp green grassy cooked apple apple skin
Luebke, William tgsc, (1990) |
| |
| Odor and/or flavor descriptions from others (if found). |
| |
| Bedoukian Research |
| cis-3-HEXEN-1-AL (50% IN TRIACETIN) ≥92.0%, Kosher |
| Odor Description: | powerful green, grassy, apple-like
Powerful and diffusive. Imparts a natural green top note to herbaceous and floral compositions. |
| Taste Description: | Sharp, green, grassy
Excellent topnote for a variety of flavors especially seedy notes for raspberry, fresh notes to apple, other fruits, and berries, and adds fresh green notes to tomato and other vegetables. |
| |
| Sigma-Aldrich |
| cis-3-Hexenal solution, 50% in triacetin, stabilized |
| Odor Description: | apple; grape; lilac; orange; floral; pear; pineapple; strawberry; green; vegetable |
| Taste Description: | apple |
| |
| |
Cosmetic Information:
Suppliers:
| Advanced Biotech |
| CIS 3 HEXENAL 1% ETOH NATURAL
|
| Advanced Biotech |
| CIS 3 HEXENAL 1% PG NATURAL
|
| Bedoukian Research |
| cis-3-HEXEN-1-AL (50% IN TRIACETIN)
≥92.0%, Kosher Odor: powerful green, grassy, apple-like Use: Powerful and diffusive. Imparts a natural green top note to herbaceous and floral compositions. Flavor: Sharp, green, grassy Excellent topnote for a variety of flavors especially seedy notes for raspberry, fresh notes to apple, other fruits, and berries, and adds fresh green notes to tomato and other vegetables. |
| Bedoukian Research |
| cis-3-HEXEN-1-AL (NEAT)
≥92.5%, Kosher Odor: powerful, green, grassy apple-like Use: Powerful and diffusive. Imparts a natural green top note to herbaceous and floral compositions. Flavor: Sharp, green, grassy Excellent topnote for a variety of flavors especially seedy notes for raspberry, fresh notes to apple, other fruits, and berries, and adds fresh green notes to tomato and other vegetables. |
| BOC Sciences |
| For experimental / research use only. |
| cis-3-HEXEN-1-AL (NEAT) 97.0% (sum of isomers)
|
| CJ Latta & Associates |
| CIS-3-HEXENAL IN CIS-3-HEXENOL 40%
|
| M&U International |
| Cis-3-Hexenal (50% in Triacetin)
|
| M&U International |
| cis-3-HEXENAL
|
| Penta International |
| CIS-3-HEXENAL (50% IN TRIACETIN)
|
| Penta International |
| CIS-3-HEXENAL 50% IN CIS-3-HEXENOL
|
| Penta International |
| CIS-3-HEXENAL NATURAL 1% IN PROPYLENE GLYCOL
|
| Penta International |
| CIS-3-HEXENAL PURE
|
| Penta International |
| CIS-3-HEXENAL SYNTHETIC 1% IN ETHYL ALCOHOL
|
| Penta International |
| CIS-3-HEXENAL SYNTHETIC 5% IN ETHYL ALCOHOL
|
| R C Treatt & Co Ltd |
| cis-3-Hexenal 50%
|
| Reincke & Fichtner |
| cis-3-Hexenal
|
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| cis-3-Hexenal
|
| Sigma-Aldrich |
| cis-3-Hexenal solution, 50% in triacetin, stabilized
Odor: apple; grape; lilac; orange; floral; pear; pineapple; strawberry; green; vegetable |
| Certified Food Grade Products |
| Synerzine |
| CIS-3-HEXENAL (NEAT)
|
| ZEON Chemicals |
| cis-3-Hexenal
|
Safety Information:
| Preferred SDS: View |
| European information : |
| Most important hazard(s): | | Xn - Harmful. |
R 10 - Flammable.
R 22 - Harmful if swallowed.
S 02 - Keep out of the reach of children.
S 16 - Keep away from sources of ignition - No Smoking.
S 20/21 - When using do not eat, drink or smoke.
S 24/25 - Avoid contact with skin and eyes.
S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S 36/37/39 - Wear suitable clothing, gloves and eye/face protection.
|
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
| None found. |
| GHS Label elements, including precautionary statements |
| |
| Pictogram | |
| |
| Hazard statement(s) |
| None found. |
| Precautionary statement(s) |
| None found. |
| Oral/Parenteral Toxicity: |
gavage-rat LD50 [sex: M/F] 1560 mg/kg
(Palanker & Lewis, 1979)
oral-rat LD50 1560 mg/kg
Food and Chemical Toxicology. Vol. 20, Pg. 709, 1982.
|
| Dermal Toxicity: |
skin-rabbit LD50 3700 mg/kg
Food and Chemical Toxicology. Vol. 20, Pg. 709, 1982.
|
| Inhalation Toxicity: |
|
Not determined
|
Safety in Use Information:
| Category: | flavor and fragrance agents |
| RIFM Fragrance Material Safety Assessment: Search |
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice |
| Recommendation for (Z)-3-hexenal usage levels up to: | | | 0.5000 % in the fragrance concentrate.
|
| |
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 4.10 (μg/capita/day) |
| Structure Class: | I |
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). |
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library |
| publication number: 3 |
| Click here to view publication 3 |
| | average usual ppm | average maximum ppm |
| baked goods: | - | - |
| beverages(nonalcoholic): | - | 0.20000 |
| beverages(alcoholic): | - | - |
| breakfast cereal: | - | - |
| cheese: | - | - |
| chewing gum: | - | - |
| condiments / relishes: | - | - |
| confectionery froastings: | - | - |
| egg products: | - | - |
| fats / oils: | - | - |
| fish products: | - | - |
| frozen dairy: | - | 5.00000 |
| fruit ices: | - | 5.00000 |
| gelatins / puddings: | - | - |
| granulated sugar: | - | - |
| gravies: | - | - |
| hard candy: | - | 5.00000 |
| imitation dairy: | - | - |
| instant coffee / tea: | - | - |
| jams / jellies: | - | - |
| meat products: | - | - |
| milk products: | - | - |
| nut products: | - | - |
| other grains: | - | - |
| poultry: | - | - |
| processed fruits: | - | - |
| processed vegetables: | - | - |
| reconstituted vegetables: | - | - |
| seasonings / flavors: | - | - |
| snack foods: | - | - |
| soft candy: | - | - |
| soups: | - | - |
| sugar substitutes: | - | - |
| sweet sauces: | - | - |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): |
Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 6 (FGE.06): Straight-and branched-chain aliphatic unsatured primary alcohols, aldehydes, carboxylic acids, and esters from chemical groups 1 and 4
View page or View pdf |
Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission related to Flavouring Group Evaluation 5: Esters of 23 branched- and straight-chain aliphatic saturated primary alcohols and of one secondary alcohol, and 24 branched- and straight-chain unsaturated carboxylic acids from chemical groups 1, 2, and 5
View page or View pdf |
Flavouring Group Evaluation 6, Revision 1 (FGE.06Rev1) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC)
View page or View pdf |
Flavouring Group Evaluation 3, Revision 1 (FGE.03Rev1): Acetals of branched- and straight-chain aliphatic saturated primary alcohols and branched- and straight-chain saturated or unsaturated aldehydes, an ester of a hemiacetal and an orthoester of formic acid, from chemical groups 1, 2 & 4 Commission Regulation (EC) No 1565/2000 of 18 July 2000) [1] - Scientific Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC)on a request from the Commission
View page or View pdf |
Flavouring Group Evaluation 5, Revision 1 (FGE.05Rev1):Esters of branched- and straight-chain aliphatic saturated primary alcohols and of one secondary alcohol, and branched- and straight-chain unsaturated carboxylic acids from chemical groups 1, 2, and 5 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) [1] - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC)
View page or View pdf |
Flavouring Group Evaluation 5, Revision 2 (FGE.05Rev2): Branched- and straight-chain unsaturated carboxylic acids and esters of these with aliphatic saturated alcohols from chemical groups 1, 2, 3 and 5
View page or View pdf |
Safety and efficacy of non-conjugated and accumulated unsaturated straight-chain and branched-chain, aliphatic primary alcohols, aldehydes, acids, acetals and esters belonging to chemical group 4 when used as flavourings for all animal species
View page or View pdf |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 6789-80-6 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 643941 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 1207 |
| WGK Germany: | 1 |
| | (Z)-hex-3-enal |
| Chemidplus: | 0006789806 |
| RTECS: | MP5940000 for cas# 6789-80-6 |
References:
Other Information:
Potential Blenders and core components note
| |
| For Odor |
| aldehydic |
| aldehydic |
| | fresh carbaldehyde | FR |
| iso | freshal | FR |
| | green hexanal | FL/FR |
| | octanal (aldehyde C-8) | FL/FR |
| | octane nitrile | FR |
| alliaceous |
| | dibutyl sulfide | FL/FR |
| balsamic |
| | guaiyl acetate | FL/FR |
| camphoreous |
| beta-homo | cyclocitral | FL/FR |
| citrus |
| | citronellal / methyl anthranilate schiff's base | FR |
| | litsea cubeba fruit oil | FL/FR |
| 2- | tetradecenal | FL/FR |
| earthy |
| 5- | cyclopropyl-3(or 2),4-dimethyl octahydro-4,7-methanoinden-5-ol | |
| ethereal |
| | ethyl acetate | FL/FR |
| 3- | decen-2-one | FL/FR |
| (E,Z)-2,6- | dodecadienal | FL/FR |
| | methyl 2-hexenoate | FL/FR |
| 6- | methyl-5-hepten-2-one propylene glycol acetal | FL/FR |
| (E)-2- | nonenal | FL/FR |
| 2- | nonenal | FL/FR |
| 2- | octenal | FL/FR |
| fermented |
| | hexanal diethyl acetal | FL/FR |
| floral |
| gamma- | damascone | FR |
| (Z)-alpha- | damascone | FL/FR |
| alpha- | damascone | FL/FR |
| | gardenia oxide | FR |
| | methyl citronellate | FL/FR |
| | mimosa absolute france | FL/FR |
| fruity |
| | acetaldehyde dihexyl acetal | FL/FR |
| | acetaldehyde hexyl isoamyl acetal | FL/FR |
| | amyl 2-methyl butyrate | FL/FR |
| | amyl isobutyrate | FL/FR |
| | amyl isovalerate | FL/FR |
| iso | amyl octanoate | FL/FR |
| | apple ketal | FL/FR |
| | benzyl isovalerate | FL/FR |
| | butyl isovalerate | FL/FR |
| | cyclohexyl butyrate | FL/FR |
| (E)-alpha- | damascone | FL/FR |
| | diethyl malonate | FL/FR |
| | eriocephalus punctulatus flower oil | FR |
| | ethyl 2-methyl butyrate | FL/FR |
| | ethyl 3,5,5-trimethyl hexanoate | FR |
| | ethyl acetoacetate | FL/FR |
| | ethyl isovalerate | FL/FR |
| | ethyl valerate | FL/FR |
| | fruity ketal | FL/FR |
| | geranyl 2-methyl butyrate | FL/FR |
| | green acetate | FR |
| (Z)-3- | hexen-1-yl isobutyrate | FL/FR |
| | hexyl (E)-tiglate | FL/FR |
| | hexyl acetate | FL/FR |
| | hexyl isovalerate | FL/FR |
| | methyl 2-methyl butyrate | FL/FR |
| | methyl 3-nonenoate | FL/FR |
| 2- | methyl butyl butyrate | FL/FR |
| | methyl isovalerate | FL/FR |
| 2- | methyl-2-pentenal | FL/FR |
| | nonyl isovalerate | FL/FR |
| | octen-1-yl cyclopentanone | FL/FR |
| | propyl 2-methyl butyrate | FL/FR |
| | propyl heptanoate | FL/FR |
| | propyl isovalerate | FL/FR |
| iso | propyl isovalerate | FL/FR |
| (E,E)-5,6,7,7- | tetramethyl-2,5-octadien-4-one | FR |
| (E)-2- | undecenal | FL/FR |
| green |
| | alfalfa absolute | FR |
| | alfalfa oil | FL/FR |
| | bark carbaldehyde | FR |
| | butyl heptanoate | FL/FR |
| sec- | butyl-3-methyl but-2-ene thioate | FL/FR |
| | cilantro leaf oil | FL/FR |
| 2,6- | dimethyl octanal | FL/FR |
| 3,7- | dimethyl-6-octenoic acid | FL/FR |
| | ethyl (E,Z)-2,4-decadienoate | FL/FR |
| | ethyl (E)-2-decenoate | FL/FR |
| | green note propionate | FL/FR |
| | heptanal (aldehyde C-7) | FL/FR |
| (Z)-3- | hepten-1-ol | FL/FR |
| 3- | hepten-2-one | FL/FR |
| (Z)-4- | heptenal | FL/FR |
| 2- | heptyl furan | FL/FR |
| | heptyl heptanoate | FL/FR |
| | hexanal (aldehyde C-6) | FL/FR |
| | hexanal dihexyl acetal | FL/FR |
| (Z)-3- | hexen-1-yl 2-methyl butyrate | FL/FR |
| (E)-2- | hexen-1-yl butyrate | FL/FR |
| (Z)-3- | hexen-1-yl butyrate | FL/FR |
| (E)-2- | hexen-1-yl propionate | FL/FR |
| (E)-2- | hexen-1-yl valerate | FL/FR |
| (Z)-3- | hexen-1-yl valerate | FL/FR |
| (E)-2- | hexenal propylene glycol acetal | FL/FR |
| | hexyl 2-methyl butyrate | FL/FR |
| | hexyl butyrate | FL/FR |
| alpha- | hexyl cinnamaldehyde dimethyl acetal | FR |
| | hexyl isobutyrate | FL/FR |
| | lilac acetaldehyde | FL/FR |
| | marigold pot absolute | FL/FR |
| 1- | methoxy-2,7-octadiene | |
| | methyl (E)-3-hexenoate | FL/FR |
| (E,Z)-3,6- | nonadien-1-ol | FL/FR |
| (Z)-2- | nonen-1-ol | FL/FR |
| (E)-2- | nonen-1-ol | FL/FR |
| 2- | nonene nitrile | FR |
| 3- | octyl formate | FL/FR |
| (E)-2- | pentenal | FL/FR |
| | phenoxyethyl isobutyrate | FL/FR |
| | propyl tiglate | FL/FR |
| | propylene acetal | FL/FR |
| 3,5,5- | trimethyl hexanol | FL/FR |
| 1,5- | undecadien-4-yl acetate | |
| herbal |
| 3- | octyl acetate | FL/FR |
| laevo- | perillaldehyde | FL/FR |
| muguet |
| 4-iso | butyl 1-(3-methoxy-2-propen-1-yl) benzene | FR |
| 4- | ethyl 1-(3-methoxy-2-propen-1-yl) benzene | FR |
| 4- | methyl 1-(3-methoxy-2-propen-1-yl) benzene | FR |
| 4- | propyl 1-(3-methoxy-2-propen-1-yl) benzene | FR |
| soapy |
| | methyl anthranilate / hexyl cinnamaldehyde schiff's base | FR |
| spicy |
| | cinnamyl isovalerate | FL/FR |
| | cumin seed absolute | FL/FR |
| 3-(2- | furyl) acrolein | FL/FR |
| waxy |
| 9- | decenoic acid | FL/FR |
| | ethyl decanoate | FL/FR |
| | heptyl octanoate | FL/FR |
| 2,4- | nonadien-1-ol | FL/FR |
| | propyl decanoate | FL/FR |
| woody |
| lariciu | pine needle oil | FR |
| |
| For Flavor |
| |
| No flavor group found for these |
| | acetaldehyde hexyl isoamyl acetal | FL/FR |
| | amyl 2-methyl butyrate | FL/FR |
| | amyl isobutyrate | FL/FR |
| | amyl isovalerate | FL/FR |
| | ascorbic acid | FL |
| sec- | butyl-3-methyl but-2-ene thioate | FL/FR |
| | cyclohexyl butyrate | FL/FR |
| 5- | cyclopropyl-3(or 2),4-dimethyl octahydro-4,7-methanoinden-5-ol | |
| (Z)-alpha- | damascone | FL/FR |
| 9- | decen-2-one | FL |
| 2- | ethyl pyridine | FL |
| | furfuryl hexanoate | FL |
| 3-(2- | furyl) acrolein | FL/FR |
| | geranyl 2-methyl butyrate | FL/FR |
| | guaiyl acetate | FL/FR |
| (E,E)-2,4- | heptadien-1-ol | FL |
| (Z)-3- | hepten-1-ol | FL/FR |
| 2- | heptenoic acid | FL |
| | hexanal butane-2,3-diol acetal | FL |
| | hexanal diethyl acetal | FL/FR |
| | hexanal dihexyl acetal | FL/FR |
| (E)-3- | hexenal | FL |
| 2- | hexenal diethyl acetal | FL |
| (E)-2- | hexenal propylene glycol acetal | FL/FR |
| (Z)-3- | hexenoic acid | FL |
| | hexyl (E)-2-hexenoate | FL |
| 2- | hexyl acetate | FL |
| 1- | methoxy-2,7-octadiene | |
| | methyl 2-hexenoate | FL/FR |
| 6- | methyl octanal | FL |
| 3-( | methyl thio) hexanal | FL |
| 6- | methyl-5-hepten-2-one propylene glycol acetal | FL/FR |
| | nonyl isovalerate | FL/FR |
| (E,E)-3,5- | octadien-2-one | FL |
| 2- | octenal | FL/FR |
| | propyl 2-methyl butyrate | FL/FR |
| | propyl decanoate | FL/FR |
| iso | propyl isovalerate | FL/FR |
| | propyl isovalerate | FL/FR |
| 2- | propyl pyridine | FL |
| | propyl tiglate | FL/FR |
| | propylene acetal | FL/FR |
| 2- | tetradecenal | FL/FR |
| 1,5- | undecadien-4-yl acetate | |
|
| (±)-3-( | methyl thio) heptanal | FL |
| aldehydic |
| | octanal (aldehyde C-8) | FL/FR |
| aromatic |
| laevo- | perillaldehyde | FL/FR |
| citrus |
| | cilantro leaf oil | FL/FR |
| | litsea cubeba fruit oil | FL/FR |
| cooling |
| beta-homo | cyclocitral | FL/FR |
| creamy |
| 3- | hepten-2-one | FL/FR |
| estery |
| | ethyl acetoacetate | FL/FR |
| ethereal |
| | ethyl acetate | FL/FR |
| fatty |
| 2,4- | decadienal | FL |
| (E,E)-2,4- | heptadienal | FL |
| 2- | heptyl furan | FL/FR |
| 2,4- | nonadien-1-ol | FL/FR |
| 2,4- | nonadienal | FL |
| (Z)-2- | nonen-1-ol | FL/FR |
| 2- | nonenal | FL/FR |
| floral |
| 3,7- | dimethyl-6-octenoic acid | FL/FR |
| | methyl citronellate | FL/FR |
| fruity |
| iso | amyl octanoate | FL/FR |
| | apple ketal | FL/FR |
| | benzyl isovalerate | FL/FR |
| | butyl heptanoate | FL/FR |
| | butyl isovalerate | FL/FR |
| | cinnamyl isovalerate | FL/FR |
| alpha- | damascone | FL/FR |
| (E)-alpha- | damascone | FL/FR |
| | diethyl malonate | FL/FR |
| | ethyl (E)-2-decenoate | FL/FR |
| | ethyl 2-methyl butyrate | FL/FR |
| | ethyl isovalerate | FL/FR |
| | ethyl valerate | FL/FR |
| | fruity ketal | FL/FR |
| (Z)-3- | hexen-1-yl isobutyrate | FL/FR |
| | hexyl acetate | FL/FR |
| | lilac acetaldehyde | FL/FR |
| | methyl (E)-3-nonenoate | FL |
| | methyl 2-methyl butyrate | FL/FR |
| | methyl 3-nonenoate | FL/FR |
| 2- | methyl butyl butyrate | FL/FR |
| | methyl isovalerate | FL/FR |
| 2- | methyl-2-pentenal | FL/FR |
| | octen-1-yl cyclopentanone | FL/FR |
| green |
| | acetaldehyde dihexyl acetal | FL/FR |
| | alfalfa oil | FL/FR |
| 3- | decen-2-one | FL/FR |
| | dibutyl sulfide | FL/FR |
| | dihydroxyacetophenone (mixed isomers) | FL |
| 2,6- | dimethyl octanal | FL/FR |
| (E,Z)-2,6- | dodecadienal | FL/FR |
| | ethyl (E,Z)-2,4-decadienoate | FL/FR |
| | green note propionate | FL/FR |
| | heptanal (aldehyde C-7) | FL/FR |
| (E)-2- | heptenal | FL |
| (Z)-4- | heptenal | FL/FR |
| | heptyl heptanoate | FL/FR |
| 2,4- | hexadienal | FL |
| | hexanal (aldehyde C-6) | FL/FR |
| (Z)-3- | hexen-1-yl 2-methyl butyrate | FL/FR |
| (E)-2- | hexen-1-yl butyrate | FL/FR |
| (Z)-3- | hexen-1-yl butyrate | FL/FR |
| (E)-2- | hexen-1-yl propionate | FL/FR |
| (E)-2- | hexen-1-yl valerate | FL/FR |
| (Z)-3- | hexen-1-yl valerate | FL/FR |
| (E)-2- | hexenal diethyl acetal | FL |
| | hexyl (E)-tiglate | FL/FR |
| | hexyl 2-methyl butyrate | FL/FR |
| | hexyl butyrate | FL/FR |
| | hexyl isobutyrate | FL/FR |
| | hexyl isovalerate | FL/FR |
| 2- | hexyl pyridine | FL |
| | marigold pot absolute | FL/FR |
| | methyl (E)-3-hexenoate | FL/FR |
| 4- | methyl-2-pentenal | FL |
| (E,Z)-3,6- | nonadien-1-ol | FL/FR |
| (E)-2- | nonen-1-ol | FL/FR |
| (E)-2- | nonenal | FL/FR |
| (E,E)-2,4- | octadienal | FL |
| 2,4- | octadienal | FL |
| 3- | octyl acetate | FL/FR |
| (E)-2- | pentenal | FL/FR |
| | phenoxyethyl isobutyrate | FL/FR |
| 3,5,5- | trimethyl hexanol | FL/FR |
| herbal |
| | green hexanal | FL/FR |
| spicy |
| | cumin seed absolute | FL/FR |
| waxy |
| 9- | decenoic acid | FL/FR |
| | ethyl decanoate | FL/FR |
| | heptyl octanoate | FL/FR |
| | mimosa absolute france | FL/FR |
| 3- | octyl formate | FL/FR |
| | propyl heptanoate | FL/FR |
| (E)-2- | undecenal | FL/FR |
| |
Potential Uses:
Occurrence (nature, food, other): noteSynonyms:
| (Z)- | hex-3-en-1-al | | (3Z)- | hex-3-enal | | (Z)- | hex-3-enal | | | hex-3(cis)-enal | | (Z)-3- | hexen-1-al | | cis-3- | hexen-1-al | | cis-3- | hexen-1-al (50% in triacetin) | | cis-3- | hexen-1-al (neat) | | (Z)-3- | hexenal | | 3-(Z)- | hexenal | | cis-3- | hexenal | | cis-3- | hexenal (50% in triacetin) | | cis-3- | hexenal pure | | 3- | hexenal, (3Z)- | | 3- | hexenal, (Z)- | | 3- | hexenal, cis- | | (Z)-beta,gamma- | hexylenic aldehyde |
Articles:
| Info: | cis-3-HEXENAL, trans-2-HEXENAL
and 'GREEN GRASS' SMELL |
| US Patents: | Derivatives of cis-3-hexenol and process for producing compositions of matter containing cis-3-hexenal and products produced thereby and organoleptic uses thereof |
| PubMed: | Key volatile aroma compounds of three black velvet tamarind (Dialium) fruit species. |
| Info: | Volatile Flavor Components in Bogyojosaeng and Suhong Cultivars of Strawberry (Fragaria ananassa Duch.) |
| US Patents: | Uses of hexenol derivatives in augementing or enhancing the aroma or taste of smoking tobacco compositions and smoking tobacco articles |
| PubMed: | Characterization of the most aroma-active compounds in cherry tomato by application of the aroma extract dilution analysis. |
| US Patents: | Compositions of matter containing cis-3-hexenal |
| PubMed: | Comparative analysis of aroma compounds and sensorial features of strawberry and lemon guavas (Psidium cattleianum Sabine). |
| PubMed: | The 9-lipoxygenase Osr9-LOX1 interacts with the 13-lipoxygenase-mediated pathway to regulate resistance to chewing and piercing-sucking herbivores in rice. |
| PubMed: | Model studies on the key aroma compounds formed by an oxidative degradation of ω-3 fatty acids initiated by either copper(II) ions or lipoxygenase. |
| PubMed: | Analyzing blends of herbivore-induced volatile organic compounds with factor analysis: revisiting "cotton plant, Gossypium hirsutum L., defense in response to nitrogen fertilization". |
| PubMed: | Influence of phenols mass fraction in olive (Olea europaea L.) paste on volatile compounds in Buža cultivar virgin olive oil. |
| PubMed: | Production of phenolics and the emission of volatile organic compounds by perennial ryegrass (Lolium perenne L.)/Neotyphodium lolii association as a response to infection by Fusarium poae. |
| PubMed: | Odour-active compounds in guava (Psidium guajava L. cv. Red Suprema). |
| PubMed: | Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-to-plant communication. |
| PubMed: | Kinetic study of the daytime atmospheric fate of (Z)-3-hexenal. |
| PubMed: | Volatile generation in bell peppers during frozen storage and thawing using selected ion flow tube mass spectrometry (SIFT-MS). |
| PubMed: | Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. |
| PubMed: | Quality evaluation of olive oil by statistical analysis of multicomponent stable isotope dilution assay data of aroma active compounds. |
| PubMed: | Gastrophysa polygoni herbivory on Rumex confertus: single leaf VOC induction and dose dependent herbivore attraction/repellence to individual compounds. |
| PubMed: | Volatile composition of four southern highbush blueberry cultivars and effect of growing location and harvest date. |
| PubMed: | Effect of enzymes on strawberry volatiles during storage, at different ripeness level, in different cultivars, and during eating. |
| PubMed: | Effect of enzyme activity and frozen storage on jalapeño pepper volatiles by selected ion flow tube-mass spectrometry. |
| PubMed: | Fusarium infection in maize: volatile induction of infected and neighboring uninfected plants has the potential to attract a pest cereal leaf beetle, Oulema melanopus. |
| PubMed: | Specific regulation of pyrethrin biosynthesis in Chrysanthemum cinerariaefolium by a blend of volatiles emitted from artificially damaged conspecific plants. |
| PubMed: | Cereal crop volatile organic compound induction after mechanical injury, beetle herbivory (Oulema spp.), or fungal infection (Fusarium spp.). |
| PubMed: | Effects of n-hexanal on dopamine release in the striatum of living rats. |
| PubMed: | Microdistillation and analysis of volatiles from eight ornamental Salvia taxa. |
| PubMed: | Relation between developmental stage, sensory properties, and volatile content of organically and conventionally grown pac choi (Brassica rapa var. Mei Qing Choi). |
| PubMed: | Comparison of volatile release in tomatillo and different varieties of tomato during chewing. |
| PubMed: | Comparison of tomatillo and tomato volatile compounds in the headspace by selected ion flow tube mass spectrometry (SIFT-MS). |
| PubMed: | The tea weevil, Myllocerinus aurolineatus, is attracted to volatiles induced by conspecifics. |
| PubMed: | Characteristic aroma-active compounds of Korean perilla (Perilla frutescens Britton) leaf. |
| PubMed: | Characterization of the key aroma compounds in pink guava (Psidium guajava L.) by means of aroma re-engineering experiments and omission tests. |
| PubMed: | In situ investigation of leaf water status by portable unilateral nuclear magnetic resonance. |
| PubMed: | Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. |
| PubMed: | Characterization of the aroma-active compounds in pink guava (Psidium guajava, L.) by application of the aroma extract dilution analysis. |
| PubMed: | Characterization of odor-active compounds in extracts obtained by simultaneous extraction/distillation from moroccan black olives. |
| PubMed: | A novel lipoxygenase gene from developing rice seeds confers dual position specificity and responds to wounding and insect attack. |
| PubMed: | Cut-induced VOC emissions from agricultural grasslands. |
| PubMed: | Genetic diversity of volatile components in Xinjiang Wild Apple (Malus sieversii). |
| PubMed: | The atmospheric photolysis of E-2-hexenal, Z-3-hexenal and E,E-2,4-hexadienal. |
| PubMed: | ETR1-, JAR1- and PAD2-dependent signaling pathways are involved in C6-aldehyde-induced defense responses of Arabidopsis. |
| PubMed: | Role of the lipoxygenase/lyase pathway of host-food plants in the host searching behavior of two parasitoid species, Cotesia glomerata and Cotesia plutellae. |
| PubMed: | Potent odorants characterize the aroma quality of leaves and stalks in raw and boiled celery. |
| PubMed: | Comparison of volatile constituents of Persicaria odorata(Lour.) Soják (Polygonum odoratum Lour.) and Persicaria hydropiper L. Spach (Polygonum hydropiper L.). |
| PubMed: | Characterization of (E,E,Z)-2,4,6-nonatrienal as a character impact aroma compound of oat flakes. |
| PubMed: | Volatile C6-aldehydes and Allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. |
| PubMed: | Rapid determination of C6-aldehydes in tomato plant emission by gas chromatography-mass spectrometry and solid-phase microextraction with on-fiber derivatization. |
| PubMed: | Screening for key odorants in Moroccan green olives by gas chromatography-olfactometry/aroma extract dilution analysis. |
| PubMed: | Some unusual minor volatile components of tomato. |
| PubMed: | Stimulation of the lipoxygenase pathway is associated with systemic resistance induced in bean by a nonpathogenic Pseudomonas strain. |
| PubMed: | Volatile constituents and key odorants in leaves, buds, flowers, and fruits of Laurus nobilis L. |
| PubMed: | Airborne signals prime plants against insect herbivore attack. |
| PubMed: | Flux of organic compounds from grass measured by relaxed eddy accumulation technique. |
| PubMed: | Volatile constituents of uncooked rhubarb (Rheum rhabarbarum L.) stalks. |
| PubMed: | Odor-active compounds of Iberian hams with different aroma characteristics. |
| PubMed: | Character impact odorants of the apple cultivars Elstar and Cox Orange. |
| PubMed: | Application of the porapak q column extraction method for tomato flavor volatile analysis. |
| PubMed: | Characterization of the most odor-active compounds of Iberian ham headspace. |
| PubMed: | The homolytic and heterolytic fatty acid hydroperoxide lyase-like activities of hematin. |
| PubMed: | On-line analysis of reactive VOCs from urban lawn mowing. |
| PubMed: | Evaluation of aroma differences between hand-squeezed juices from Valencia late and Navel oranges by quantitation of key odorants and flavor reconstitution experiments. |
| PubMed: | Potato tubers exhibit both homolytic and heterolytic hydroperoxide fatty acid-cleaving activities. |
| PubMed: | Comparison of the most odor-active compounds in fresh and dried hop cones (Humulus lupulus L. variety spalter select) based on GC-olfactometry and odor dilution techniques. |
| PubMed: | Characterization of the most odor-active volatiles in fresh, hand-squeezed juice of grapefruit (Citrus paradisi Macfayden). |
| PubMed: | Aroma chemicals isolated and identified from leaves of Aloe arborescens Mill. Var. Natalensis Berger. |
| PubMed: | Overexpression of a cytoplasm-localized allene oxide synthase promotes the wound-induced accumulation of jasmonic acid in transgenic tobacco. |
| PubMed: | Molecular cloning and expression of Arabidopsis fatty acid hydroperoxide lyase. |
| PubMed: | Characterization of aroma volatiles in tomatoes by sensory analyses. |
| PubMed: | Photosynthetic photon flux, photoperiod, and temperature effects on emissions of (Z)-3-hexenal, (Z)-3-hexenol, and (Z)-3-hexenyl acetate from lettuce. |
| PubMed: | A system and methodology for measuring volatile organic compounds produced by hydroponic lettuce in a controlled environment. |
| PubMed: | Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. |
| PubMed: | Isolation and identification of allelochemicals that attract the larval parasitoid,Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. |
|
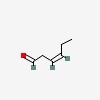 3D/inchi
3D/inchi








