Articles:
3-buten-2-one, 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-, (3E)-
Notes:
Leaf effects with mhc.
new car accord:
0.01 - dimethyl benzyl carbinyl acetate
0.01 - alpha-ionone
0.05 - heptyl cyclopentanone
0.01 - musk gx
0.05 - aldehyde C-14
0.01 - dihydroisojasmonate
| Fragrance Demo Formulas Flavor Demo Formulas | ||
| CAS Number: | 127-41-3 | 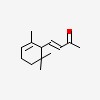 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 30685-95-1 | |
| % from: | 79.00% to 94.00% | |
| ECHA EINECS - REACH Pre-Reg: | 204-841-6 | |
| FDA UNII: | I9V075M61R | |
| Nikkaji Web: | J183.660I | |
| Beilstein Number: | 3197885 | |
| MDL: | MFCD00001565 | |
| CoE Number: | 141 | |
| XlogP3-AA: | 3.00 (est) | |
| Molecular Weight: | 192.30160000 | |
| Formula: | C13 H20 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
| Also(can) Contains: | beta-ionone 4.00% to 20.00% | |
| gamma-ionone 1.00% to 8.00% | ||
| pseudoionone 0.10% to 1.00% | ||
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 388 alpha-ionone |
| DG SANTE Food Flavourings: | 07.007 alpha-ionone |
| FEMA Number: | 2594 alpha-ionone |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 127-41-3 ; ALPHA-IONONE |
| FDA Regulation: | |
| FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION Subpart F--Flavoring Agents and Related Substances Sec. 172.515 Synthetic flavoring substances and adjuvants. | |
Physical Properties:
| Appearance: | colorless to pale yellow clear liquid (est) |
| Assay: | 85.00 to 100.00 % sum of isomers |
| Additional Assay Information: | min 95% sum of isomers |
| Food Chemicals Codex Listed: | Yes |
| Specific Gravity: | 0.92700 to 0.93300 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 7.714 to 7.763 |
| Specific Gravity: | 0.92800 to 0.93600 @ 20.00 °C. |
| Pounds per Gallon - est.: | 7.731 to 7.798 |
| Refractive Index: | 1.49700 to 1.50200 @ 20.00 °C. |
| Boiling Point: | 131.00 °C. @ 13.00 mm Hg |
| Boiling Point: | 237.00 to 238.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.014000 mmHg @ 25.00 °C. (est) |
| Vapor Density: | 7 ( Air = 1 ) |
| Flash Point: | > 230.00 °F. TCC ( > 110.00 °C. ) |
| logP (o/w): | 3.995 |
| Shelf Life: | 24.00 month(s) or longer if stored properly. |
| Storage: | refrigerate in tightly sealed containers. |
| Soluble in: | |
| alcohol | |
| dipropylene glycol | |
| fixed oils | |
| paraffin oil | |
| water, 1.00E+06 mg/L @ 25 °C (exp) | |
| Insoluble in: | |
| glycerin | |
| Stability: | |
| acid cleaner | |
| alcoholic lotion | |
| antiperspirant | |
| deo stick | |
| detergent perborate | |
| fabric softener | |
| hard surface cleaner | |
| liquid detergent | |
| non-discoloring in most media | |
| shampoo | |
| soap | |
Organoleptic Properties:
| Odor Type: floral | |
| Odor Strength: | medium , recommend smelling in a 10.00 % solution or less |
| Substantivity: | 112 hour(s) at 100.00 % |
| sweet woody floral violet orris tropical fruity | |
| Odor Description: at 10.00 % in dipropylene glycol. | sweet woody floral violet orris tropical fruity Luebke, William tgsc, (1983) |
| Flavor Type: floral | |
| floral violet powdery berry raspberry tropical blackberry blueberry | |
| Taste Description: | floral violet powdery berry raspberry tropical blackberry blueberry Luebke, William tgsc, (1983) |
| Odor and/or flavor descriptions from others (if found). | |
| Givaudan | |
| Irisone Alpha | |
| Odor Description: | sweet floral violet raspberry Irisone Alpha is used widely in perfume compositions of almost all types. The use of Irisone Alpha in rose bases is very common. It is used in woody, floral, balsamic, piney or citrus-like fragrances. Good substantivity. |
| Bedoukian Research | |
| alpha-IONONE BRI ≥91.0%, FCC, Kosher | |
| Odor Description: | A high purity alpha ionone with a clean, warm violet, woody Lends a violet/berry note to fragrances. Used in rose bases. |
| Taste Description: | Characteristic ionone, floral, berry Useful in all types of berry flavors, especially raspberry and blackberry, and imparts cherry-floral notes for a variety of flavors. Also useful for honey and tea flavors. |
| IFF | |
| Ionone Alpha | |
| Odor Description: | A fine quality of alpha ionone. This is the most floral and most violet-like of all the ionones |
| Taste Description: | violet |
| Takasago | |
| Ionone Alpha ≥70% as alpha-Ionone | |
| Odor Description: | Warm-woody, balsamic-floral Used in many floral accords. Gives a violet flower nuance to compositions, but is also used in rose, woody, amber, balsamic, and powdery fragrances. |
| Alfrebro | |
| alpha-IONONE NATURAL 50% IN ETHANOL | |
| Odor Description: | Warm, Violet, Woody, Berry |
| Moellhausen | |
| alpha-IONONE 70% | |
| Odor Description: | floreal, cedar wood, in dilution violet and orris note |
| Taste Description: | warm, raspberry, strawberry, floreal |
| Moellhausen | |
| Alpha-IONONE 90% | |
| Odor Description: | floreal, cedar wood, in dilution violet and orris note |
| Taste Description: | warm, raspberry, strawberry, floreal |
| PerfumersWorld | |
| alpha-Ionone | |
| Odor Description: | woody floral violet flowers violet Warm-woody balsamic-Iloral BLENDS WITH - Ionone Balsams |
| Taste Description: | violet |
| SRS Aromatics | |
| ALPHA IONONE 90 | |
| Odor Description: | Sweet, Woody, Floral, Violet, Fruity |
| Taste Description: | violet |
| Pell Wall Perfumes | |
| Ionone alpha | |
| Odor Description: | Floral-violet, sweet, warm-woody, balsamic-floral The most floral and violet of the ionones, that is, as Arctander points out: “used widely in perfume compositions of almost all types. It is many decades since the single floral Violet note was appreciated as a fragrance for cosmetic purposes, but lonone has found numerous other applications. The use of lonone in Rose bases is very common and generally well liked, and smaller amounts of lonone are used in woody, herbaceous, floral, balsamic, piney or Citrus-like fragrances. It is almost not possibe to name a fragrance in which Ionone has not been tried for modifications, blending, floralizing mellowing etc. It is often part of the highly desirable complex that displays �powdery’ undertones in a fragrance.“ |
| Taste Description: | violet |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents |
Suppliers:
| Advanced Biotech |
| ALPHA IONONE 50% ETOH NATURAL
Odor: Alcoholic, Berry, Violet |
| Advanced Biotech |
| ALPHA IONONE NATURAL
90% min. (mixed isomers) Odor: Berry, Violet, Woody |
| Advanced Biotech |
| IONONE (S TYPE) NATURAL |
| Alfrebro |
| alpha-IONONE NATURAL (1 kg) |
| Alfrebro |
| alpha-IONONE NATURAL (5 kg)
Odor: Warm, Woody, Violet, Floral |
| Alfrebro |
| alpha-IONONE NATURAL 10% IN ETHANOL
Odor: Warm, Violet, Woody, Berry |
| Alfrebro |
| alpha-IONONE NATURAL 50% IN ETHANOL
Odor: Warm, Violet, Woody, Berry |
| Artiste |
| alpha-Ionone Natural |
| Augustus Oils |
| Ionone Alpha |
| Services |
| Aurochemicals |
| IONONE ALPHA, Natural |
| Azelis UK |
| IONONE ALPHA BHT |
| Bedoukian Research |
| alpha-IONONE BRI
≥91.0%, FCC, Kosher Odor: A high purity alpha ionone with a clean, warm violet, woody Use: Lends a violet/berry note to fragrances. Used in rose bases. Flavor: Characteristic ionone, floral, berry Useful in all types of berry flavors, especially raspberry and blackberry, and imparts cherry-floral notes for a variety of flavors. Also useful for honey and tea flavors. |
| Beijing Lys Chemicals |
| Ionone apha |
| Beijing Lys Chemicals |
| Natural ionone alpha |
| Berjé |
| Ionone Alpha 70 |
| Media |
| Berjé |
| Ionone Alpha 80 |
| BOC Sciences |
| For experimental / research use only. |
| alpha-IONONE BRI FCC 95.0% (sum of isomers) |
| Citrus and Allied Essences |
| alpha-Ionone (natural) |
| Market Report |
| CJ Latta & Associates |
| ALFA IONONE |
| CJ Latta & Associates |
| Nat.Alpha Ionone |
| Creatingperfume.com |
| alpha-Ionone White Coeur
Odor: Warm,woody, balsamic, floral |
| Diffusions Aromatiques |
| IONONE ALPHA |
| Diffusions Aromatiques |
| IONONE FOOD GRADE |
| ECSA Chemicals |
| ALFA IONONE 95 |
| ECSA TRADE THE MOST UPDATED FINANCIAL PUBLICATION ON THE WORLD OF CHEMISTRY |
| ECSA Chemicals |
| ALPHA IONONE 70 |
| Ernesto Ventós |
| IONONE 100% IFF
Odor: WOODY, WARM, VIOLET |
| Ernesto Ventós |
| IONONE ALPHA (FOOD GRADE E.U.)
Odor: WOODY, FLORAL, VIOLET, FRUITY |
| Ernesto Ventós |
| IONONE ALPHA 50% NATURAL
Odor: SWEETISH, BERRY, RASPBERRY |
| Ernesto Ventós |
| IONONE ALPHA, NATURAL |
| Ernesto Ventós |
| IONONE ALPHA
Odor: WOODY,WARM, VIOLET |
| ExtraSynthese |
| For experimental / research use only. |
| alpha-Ionone |
| Fleurchem |
| alpha-ionone natural |
| Fleurchem |
| alpha-ionone |
| Foreverest Resources |
| Ionone Alpha
Odor: sweet woody floral violet orris tropical fruity Use: The ionones are a series of closely related chemical substances.They are part of rose ketones, which also includes damascones and damascenones. |
| Frutarom |
| NATURAL ALPHA IONONE
KOSHER Flavor: Sweet, Woody, Floral, Violet, Tropical |
| CBD Offering |
| Fuzhou Farwell |
| Alpha-lonone |
| Givaudan |
| Irisone Alpha
Odor: sweet floral violet raspberry Use: Irisone Alpha is used widely in perfume compositions of almost all types. The use of Irisone Alpha in rose bases is very common. It is used in woody, floral, balsamic, piney or citrus-like fragrances. Good substantivity. |
| Global Essence |
| alpha-Ionone Natural |
| Global Essence |
| Ionone Alpha |
| IFF |
| Ionone Alpha
Odor: A fine quality of alpha ionone. This is the most floral and most violet-like of all the ionones |
| IFF |
| NATURAL ALPHA IONONE
KOSHER Flavor: Sweet, Woody, Floral, Violet, Tropical |
| Indukern F&F |
| IONONE ALPHA Food Grade
Odor: WOODY, WARM, VIOLET |
| Indukern F&F |
| IONONE ALPHA
Odor: WOODY, WARM, VIOLET |
| Jiangyin Healthway |
| a-Ionone |
| New functional food ingredients |
| Jiangyin Healthway |
| alpha-Ionone Natural85% |
| K.L. Koh Enterprise |
| IONONE ALPHA |
| Keva |
| IONONE ALPHA |
| Kun Shan P&A |
| Ionone Alpha |
| Lluch Essence |
| ALPHA-IONONE NATURAL |
| Lluch Essence |
| ALPHA-IONONE |
| M&U International |
| alpha-IONONE |
| M&U International |
| Ionone Alpha Regular |
| M&U International |
| Ionone Alpha |
| M&U International |
| Ionone Extra |
| M&U International |
| NAT.ALPHA-IONONE, Kosher |
| Miltitz Aromatics |
| ALPHA-IONONE 70
min. 69 % Odor: Sweet, warm, balsamic, floral, in dilution violet-like with suggestion of orris and cedarwood Use: Alpha Ionone is widely used in nearly all perfum types, like rosy, balsamic, woody, floral and also citrusy compositions. |
| Miltitz Aromatics |
| ALPHA-IONONE 80
min. 79 % Odor: Sweet, warm, balsamic, floral, in dilution violet-like with suggestion of orris and cedarwood Use: Alpha Ionone is widely used in nearly all perfum types, like rosy, balsamic, woody, floral and also citrusy compositions. |
| Miltitz Aromatics |
| ALPHA-IONONE 90
min. 88 % Odor: Sweet, warm, balsamic, floral, in dilution violet-like with suggestion of orris and cedarwood Use: Alpha Ionone is widely used in nearly all perfum types, like rosy, balsamic, woody, floral and also citrusy compositions. |
| Moellhausen |
| alpha-IONONE 70%
Odor: floreal, cedar wood, in dilution violet and orris note Flavor: warm, raspberry, strawberry, floreal |
| Moellhausen |
| Alpha-IONONE 90%
Odor: floreal, cedar wood, in dilution violet and orris note Flavor: warm, raspberry, strawberry, floreal |
| Natural Advantage |
| Ionone, Alpha Nat High Purity
Flavor: balsamic, fruity, raspberry, violet, woody |
| Riverside Aromatics LTD.is the exclusive distributor for Europe in UK for any non-US based inquiries |
| Natural Advantage |
| Ionone, Alpha Nat
Flavor: balsamic, fruity, raspberry, violet, woody |
| O'Laughlin Industries |
| ALPHA IONONE >90% |
| Omega Ingredients |
| alpha Ionone Natural >95% |
| Omega Ingredients |
| alpha Ionone Natural 50% in Ethanol |
| OQEMA |
| Ionone 100% |
| OQEMA |
| Ionone Alpha natural |
| OQEMA |
| Ionone Alpha |
| PCW France |
| Ionone Alpha |
| Steps to a fragranced product |
| Pell Wall Perfumes |
| Ionone alpha
Odor: Floral-violet, sweet, warm-woody, balsamic-floral Use: The most floral and violet of the ionones, that is, as Arctander points out: “used widely in perfume compositions of almost all types. It is many decades since the single floral Violet note was appreciated as a fragrance for cosmetic purposes, but lonone has found numerous other applications. The use of lonone in Rose bases is very common and generally well liked, and smaller amounts of lonone are used in woody, herbaceous, floral, balsamic, piney or Citrus-like fragrances. It is almost not possibe to name a fragrance in which Ionone has not been tried for modifications, blending, floralizing mellowing etc. It is often part of the highly desirable complex that displays �powdery’ undertones in a fragrance.“ |
| Penta International |
| ALPHA IONONE NATURAL |
| Penta International |
| ALPHA IONONE REFINED |
| Penta International |
| ALPHA IONONE REGULAR |
| Penta International |
| IONONE ALPHA BETA |
| Penta International |
| IRISONE ALPHA EXTRA WHITE |
| PerfumersWorld |
| alpha-Ionone
Odor: woody floral violet flowers violet Warm-woody balsamic-Iloral Use: BLENDS WITH - Ionone Balsams |
| Perfumery Laboratory |
| ALPHA IONON WHITE IFF (Alpha Ionone White Coeur IFF)
Odor: Sweet, clean, warm, balsamic, floral violet |
| Perfumery Laboratory |
| IONON ALPHA IFF (IONONE ALPHA IFF)
Odor: Pure and sweet violet note. Has a small woody tone |
| Perfumery Laboratory |
| IONON ALPHA natural (IONONE ALPHA natural)
Odor: Pure and sweet violet note |
| Phoenix Aromas & Essential Oils |
| alpha-Ionone Natural |
| Phoenix Aromas & Essential Oils |
| Alpha-Ionone |
| Privi Organics |
| alpha-Ionone 950 |
| Privi Organics |
| alpha-Ionone |
| Prodasynth |
| ALPHA IONONE
(> 70%) Odor: WOODY,WARM, VIOLET |
| Reincke & Fichtner |
| alpha-Ionone natural |
| Reincke & Fichtner |
| alpha-Ionone |
| Riverside Aromatics |
| alpha-IONONE, NATURAL |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| alpha-Ionone ≥96% |
| Sigma-Aldrich |
| a-Ionone, ≥90%
Odor: berry; cherry; woody; raspberry; violet |
| Certified Food Grade Products |
| Sigma-Aldrich |
| a-Ionone, natural, ≥84%
Odor: berry; cherry; woody; raspberry; violet |
| SRS Aromatics |
| ALPHA IONONE 70
Odor: Sweet, Woody, Floral, Violet, Fruity |
| SRS Aromatics |
| ALPHA IONONE 80
Odor: Sweet, Woody, Floral, Violet, Fruity |
| SRS Aromatics |
| ALPHA IONONE 90
Odor: Sweet, Woody, Floral, Violet, Fruity |
| SRS Aromatics |
| ALPHA IONONE EU NATURAL |
| SRS Aromatics |
| ALPHA IONONE
Odor: Sweet, Woody, Floral, Violet, Fruity |
| Sunaux International |
| nat.alpha-Ionone |
| Synerzine |
| ALPHA IONONE, NATURAL |
| Synerzine |
| ALPHA IONONE |
| Takasago |
| Ionone Alpha
≥70% as alpha-Ionone Odor: Warm-woody, balsamic-floral Use: Used in many floral accords. Gives a violet flower nuance to compositions, but is also used in rose, woody, amber, balsamic, and powdery fragrances. |
| The Fragrance Museum |
| Taytonn ASCC |
| Ionone Alpha |
| TCI AMERICA |
| For experimental / research use only. |
| alpha-Ionone >90.0%(GC) |
| The Good Scents Company |
| alpha-ionone
Odor: sweet woody floral violet orris tropical fruity |
| The John D. Walsh Company |
| Ionone Alpha Regular |
| The John D. Walsh Company |
| Ionone Alpha White Coeur |
| The Lermond Company |
| IONONE ALPHA, NATURAL |
| The Lermond Company |
| IONONE ALPHA |
| The Perfumers Apprentice |
| alpha-Ionone (Natural) |
| The Perfumers Apprentice |
| Ionone alpha (Ionone Alpha White Coeur) (I)
Odor: orris note - classic violet/Berry, sweet ionone like with a woody floral powdery note |
| Tianjin Danjun International |
| �-Ionone |
| Ungerer & Company |
| alpha-Ionone Refined |
| Vigon International |
| Ionone Alpha 60% (Irisone Pure)
Odor: FLORAL ODORS, PRIMARILY OF A VIOLET CHARACTER, SOMEWHAT ON THE WOODY-FRUITY SIDE |
| Vigon International |
| Ionone Alpha 90% (Irisone Alpha)
Odor: Warm woody violet orris floral |
| WEN International |
| ALPHA-IONONE Natural |
| WholeChem |
| Ionone apha |
| Zanos |
| Alpha Ionone
Odor: Floral, Woody, Orris Fruity, Violet |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xi - Irritant | |
|
R 36/38 - Irritating to skin and eyes. R 42/43 - May cause sensitization by inhalation and skin contact. S 02 - Keep out of the reach of children. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 36 - Wear suitable protective clothing. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| Respiratory sensitisation (Category 1), H334 | |
| GHS Label elements, including precautionary statements | |
| Pictogram |  |
| Signal word | Danger |
| Hazard statement(s) | |
| H334 - May cause allergy or asthma symptoms or breathing difficulties if inhaled | |
| Precautionary statement(s) | |
| P261 - Avoid breathing dust/fume/gas/mist/vapours/spray. P285 - In case of inadequate ventilation wear respiratory protection. P304 + P341 - IF INHALED: If breathing is difficult, remove victim to fresh air and keep at rest in a position comfortable for breathing. P342 + P311 - IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. P501 - Dispose of contents/ container to an approved waste disposal plant. | |
| Oral/Parenteral Toxicity: | |
|
intraperitoneal-mouse LD50 2277 mg/kg FAO Nutrition Meetings Report Series. Vol. 44A, Pg. 46, 1967. | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| IFRA Critical Effect: | Sensitization | ||
| IFRA Other Specification: | <= 2% Pseudoionone | ||
| maximum skin levels for fine fragrances: | |||
| 1.0000 % and are based on the assumption that the fragrance mixture is used at 20% in a consumer product (IFRA Use Level Survey). (IFRA, 2002) | |||
| Recommendation for alpha-ionone usage levels up to: | |||
| 15.0000 % in the fragrance concentrate. | |||
| use level in formulae for use in cosmetics: | |||
| 2.0100 % | |||
| Dermal Systemic Exposure in Cosmetic Products: | |||
| 0.05 mg/kg/day (IFRA, 2002) | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 270.00 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 150.00 (μg/capita/day) | ||
| Structure Class: | I | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 3 | |||
| Click here to view publication 3 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | 6.70000 | |
| beverages(nonalcoholic): | - | 2.50000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | 39.00000 | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | 50.00000 | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | 3.60000 | |
| fruit ices: | - | 3.60000 | |
| gelatins / puddings: | - | 3.60000 | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | 12.00000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of alicyclic substances used as flavor ingredients. View pdf | |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Flavouring Group Evaluation 213: alpha,beta-Unsaturated alicyclic ketones and precursors from chemical subgroup 2.7 of FGE.19 View page or View pdf | |
| List of apha, beta-Unsaturated Aldehydes and Ketones representative of FGE.19 substances for Genotoxicity Testing [1] - Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) View page or View pdf | |
| Flavouring Group Evaluation 210: alpha,beta-Unsaturated alicyclic ketones and precursors from chemical subgroup 2.4 of FGE.19[1] View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 210 Revision 2 (FGE.210Rev2): Consideration of genotoxic potential for a,�-unsaturated alicyclic ketones and precursors from chemical subgroup 2.4 of FGE.19 View page or View pdf | |
| Safety and efficacy of secondary alicyclic saturated and unsaturated alcohols, ketones, ketals and esters with ketals containing alicyclic alcohols or ketones and esters containing secondary alicyclic alcohols from chemical group 8 when used as flavourings for all animal species View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 210 Revision 3 (FGE.210Rev3): Consideration of genotoxic potential for a,�-unsaturated alicyclic ketones and precursors from chemical subgroup 2.4 of FGE.19 View page or View pdf | |
| Safety of 31 flavouring compounds belonging to different chemical groups when used as feed additives for all animal species View page or View pdf | |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 127-41-3 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 5282108 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 2 |
| (E)-4-(2,6,6-trimethyl-1-cyclohex-2-enyl)but-3-en-2-one | |
| Chemidplus: | 0000127413 |
| RTECS: | EN0525000 for cas# 127-41-3 |
References:
| Leffingwell: | Chirality or Article |
| (E)-4-(2,6,6-trimethyl-1-cyclohex-2-enyl)but-3-en-2-one | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 127-41-3 |
| Pubchem (cid): | 5282108 |
| Pubchem (sid): | 134974059 |
| Flavornet: | 127-41-3 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C12286 |
| HMDB (The Human Metabolome Database): | HMDB59883 |
| FooDB: | FDB014484 |
| Export Tariff Code: | 2914.23.0000 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
Potential Uses:
| abronia | FR | |
| acacia | FR | |
| aimant | ||
| almond | FR | |
| amber | FR | |
| ambrene | FR | |
| angel essence | FR | |
| apple blossom | FR | |
| apricot | FR | |
| azalea | FR | |
| balsam | FR | |
| banana | FR | |
| bergamot | FR | |
| berry | FR | |
| blackberry | FR | |
| blueberry | FR | |
| boronia | FR | |
| cedar | FR | |
| chanel #5 | ||
| cherry | FR | |
| cherry black cherry | FR | |
| cherry blossom | FR | |
| christmas | FR | |
| citrus | FR | |
| country meadow | FR | |
| crabapple blossom | FR | |
| currant | FR | |
| currant bud absolute replacer | FL/FR | |
| emeraude | ||
| fir | ||
| fixer | ||
| floral | FR | |
| florida breeze | FR | |
| gardenia | FR | |
| genet | FR | |
| grape | FR | |
| hibiscus | FR | |
| honey | FR | |
| hugonia | FR | |
| hyacinth | FR | |
| jasmin | FR | |
| l'heure bleue | ||
| l'origan | ||
| leaf | ||
| lemon | FR | |
| lily | FR | |
| lily of the valley | FR | |
| linden flower | FR | |
| loganberry | FR | |
| lotus | FR | |
| mulberry | FR | |
| musk | FR | |
| opoponax | FR | |
| oriental | FR | |
| orris | FR | |
| papaya | FR | |
| paris | ||
| passion fruit | FR | |
| pine | FR | |
| plum | FR | |
| poppy red poppy | FR | |
| powder | FR | |
| raspberry | FR | |
| rose | FR | |
| rose red rose | FR | |
| rose tea rose | FR | |
| rose white rose | FR | |
| sandalwood | FR | |
| scandal | ||
| strawberry | FR | |
| sweet pea | FR | |
| tabu | ||
| tide | ||
| tropical | FL | |
| tuberose | FR | |
| valerian | FL/FR | |
| violet | FR | |
| wallflower | FR | |
| woody | FR | |
| ylang ylang | FR |
Occurrence (nature, food, other): note
| acacia sweet acacia Search Trop Picture | |
| almond roasted almond Search Trop Picture | |
| blackberry fruit Search PMC Picture | |
| brandy grape brandy Search PMC Picture | |
| carrot Search Trop Picture | |
| champaca concrete @ 0.03% Data GC Search Trop Picture | |
| currant black currant fruit Search Trop Picture | |
| plum fruit Search Trop Picture | |
| plumcot fruit Search PMC Picture | |
| raspberry red raspberry fruit Search Trop Picture | |
| rum Search PMC Picture | |
| sphaeranthus indicus Search Trop Picture | |
| tea Search Trop Picture | |
| tobacco burley tobacco Search Picture | |
| whiskey Search Picture | |
| wine Search Picture |
Synonyms:
| alphaline 70 | |
| 3- | buten-2-one, 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-, (3E)- |
| (E)-alpha- | cyclocitrylidene acetone |
| trans-alpha- | cyclocitrylidene acetone |
| ionanthem | |
| (±)-(E)-alpha- | ionone |
| (±)-trans-alpha- | ionone |
| (E)-a- | ionone |
| (E)-alpha- | ionone |
| a- | ionone |
| nat.alpha- | ionone |
| trans-alpha- | ionone |
| alpha- | ionone (natural) |
| alpha- | ionone 50% in ETOH natural |
| alpha- | ionone 60 |
| alpha- | ionone 70 |
| alpha- | ionone 80 |
| alpha- | ionone 90 |
| alpha- | ionone 90%, (naturals) |
| ionone alpha | |
| ionone alpha regular | |
| ionone alpha white coeur | |
| alpha- | ionone BRI |
| alpha- | ionone extra |
| alpha- | ionone natural |
| alpha- | ionone refined |
| alpha- | ionone white coeur |
| alpha- | ionone, natural |
| alpha- | ionone, refined |
| alpha- | ionone, regular |
| irisone alpha | |
| irisone alpha extra white | |
| (E)-4-(2,6,6- | trimethyl-1-cyclohex-2-enyl)but-3-en-2-one |
| (E)-(±)-4,2,6,6- | trimethyl-2-cyclohexen-1-yl-3-buten-2-one |
| trans-4,2,6,6- | trimethyl-2-cyclohexen-1-yl-3-buten-2-one |
| (3E)-4-(2,6,6- | trimethyl-2-cyclohexen-1-yl)-3-buten-2-one |
| (E)-(±)-4-(2,6,6- | trimethyl-2-cyclohexen-1-yl)-3-buten-2-one |
| (E)-(1)-4-(2,6,6- | trimethyl-2-cyclohexen-1-yl)-3-buten-2-one |
| (E)-4-(2,6,6- | trimethyl-2-cyclohexen-1-yl)-3-buten-2-one |
| (3E)-(±)-4-(2,6,6- | trimethyl-4-cyclohexen-1-yl)-3-buten-2-one |
| (3E)-4-(2,6,6- | trimethylcyclohex-2-en-1-yl)but-3-en-2-one |
| (3E)-4-(2,6,6- | trimethylcyclohex-2-enyl)but-3-en-2-one |










































