Articles:
isopsoralen
Notes:
used as tranquillizer; sedative; or anticonvulsant. Constit. of roots and leaves of angelica (Angelica archangelica). Found in roots and on surface of parsnips and diseased celery
Angelicin is a furanocoumarin. It can be found in Bituminaria bituminosa. It is present in the list of IARC Group 3 carcinogens (Angelicin plus ultraviolet A radiation). (Wikipedia)
| CAS Number: | 523-50-2 | 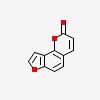 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 39310-13-9 | |
| FDA UNII: | CZZ080D7BD | |
| Nikkaji Web: | J1.579B | |
| Beilstein Number: | 0153970 | |
| MDL: | MFCD00064930 | |
| XlogP3: | 2.00 (est) | |
| Molecular Weight: | 186.16642000 | |
| Formula: | C11 H6 O3 | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 138.00 to 139.50 °C. @ 760.00 mm Hg |
| Boiling Point: | 360.00 to 362.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.000100 mmHg @ 25.00 °C. |
| Flash Point: | 343.00 °F. TCC ( 172.78 °C. ) |
| logP (o/w): | 2.080 |
| Shelf Life: | 12.00 month(s) or longer if stored properly. |
| Storage: | refrigerate in tightly sealed containers. |
| Soluble in: | |
| alcohol | |
| water, 859.6 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
| Similar Items: note | |
| psoralen | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Isopsoralen 98% |
| BOC Sciences |
| For experimental / research use only. |
| Isopsoralen >95%
Odor: characteristic Use: Angelicin is a natural furocoumarin isolated from Psoralea corylifolia L. fruit, |
| Carbosynth |
| For experimental / research use only. |
| Angelicin |
| Coompo |
| For experimental / research use only. |
| Angelicin from Plants ≥96%
Odor: characteristic Use: Angelicin displays a wide variety of potent and interesting biological activities. These include antifungal activities, inducing S-phase delay in the rad 14?mutant, inhibition of mutagenesis of 2-amino-3-methylimidazo[4,5-f]quinoloine, inhibitory effects on the biotransformation of aflatoxin B1 to aflatoxin B1-8,9-epoxide, and inhibition of inducible nitric oxide synthase.
Angelicin is structurally related to psoralens, a well-known chemical class of photosensitizers used for its antiproliferative activity in treatment of different skin diseases. To verify the activity of angelicin, we employed human SH-SY5Y neuroblastoma cells to investigate its cytotoxicity, although its mechanism of action has not yet been fully elucidated. Here, we examined the cellular cytotoxicity of angelicin by cell viability assay, DNA fragmentation by DNA ladder assay, and activation of caspases and Bcl-2 family proteins by western blot analyses. The results of our investigation suggest that angelicin increased cellular cytotoxicity in a dose- and time-dependent manner with IC(50) of 49.56 �M at 48 h of incubation. In addition, angelicin dose-dependently downregulated the expression of anti-apoptotic proteins including Bcl-2, Bcl-xL, and Mcl-1 suggesting the involvement of the intrinsic mitochondria-mediated apoptotic pathway which did not participate in Fas/FasL-induced caspase-8-mediated extrinsic, MAP kinases, and PI3K/AKT/GSK-3β pathway. Furthermore, we clarified the dose-dependent upregulation of caspase-9 and caspase-3 which indicated that angelicin-induced apoptosis is mediated primarily through the intrinsic caspase-mediated pathway. In particular, the caspase-3 inhibitor, DEVD-fmk, induced a reduction in angelicin-induced cytotoxicity which confirmed that the intrinsic caspase-dependent pathway during this apoptosis which did not prevent cytotoxicity using MAP kinases and GSK-3 inhibitor. Taken together, our data shows that angelicin is an effective apoptosis-inducing natural compound of human SH-SY5Y neuroblastoma cells which suggests that this compound may have a role in future therapies for human neuroblastoma cancer. |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| Angelicin |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| Angelicin |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 20/21/22 - Harmful by inhalation, in contact with skin and if swallowed. R 36/37/38 - Irritating to eyes, respiratory system, and skin. R 40 - Possible risks of irreversable effects. S 02 - Keep out of the reach of children. S 22 - Do not breath dust. S 23 - Do not breath vapour. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 36/37/39 - Wear suitable clothing, gloves and eye/face protection. S 45 - In case of accident or if you feel unwell seek medical advice immediately. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 322 mg/kg BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: ANALGESIA BEHAVIORAL: ATAXIA Indian Journal of Medical Research. Vol. 63, Pg. 833, 1975. intraperitoneal-rat LD50 165 mg/kg BEHAVIORAL: ANALGESIA BEHAVIORAL: ATAXIA BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) Indian Journal of Medical Research. Vol. 63, Pg. 833, 1975. intraperitoneal-mouse LD50 254 mg/kg BEHAVIORAL: ATAXIA BEHAVIORAL: ANALGESIA BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) Indian Journal of Medical Research. Vol. 63, Pg. 833, 1975. | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for angelicin usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for angelicin flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA GENetic TOXicology: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 10658 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| furo[2,3-h]chromen-2-one | |
| Chemidplus: | 0000523502 |
| RTECS: | LV0940000 for cas# 523-50-2 |
References:
| furo[2,3-h]chromen-2-one | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 10658 |
| Pubchem (sid): | 134977795 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C09060 |
| HMDB (The Human Metabolome Database): | HMDB33930 |
| FooDB: | FDB012132 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| EFSA Update of results on the monitoring of furan levels in food: | Read Report |
| EFSA Previous report: Results on the monitoring of furan levels in food: | Read Report |
| EFSA Report of the CONTAM Panel on provisional findings on furan in food: | Read Report |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| angelica root oil Search Trop Picture | |
| celery stem Search Trop Picture | |
| coriander fruit Search Trop Picture | |
| coriander seed Search Trop Picture | |
| fig flower Search Trop Picture | |
| fig fruit Search Trop Picture | |
| heracleum paphlagonicum czeczott fruit oil turkey @ 0.10% Data GC Search Trop Picture | |
| parsnip root Search Trop Picture | |
| rue oil china @ 0.36% Data GC Search Trop Picture |
Synonyms:
| angecin | |
| 2-oxo-(2H)- | furo(2,3-H)-1-benzopyran |
| furo(2,3-H)coumarin | |
| furo(5',4':7,8)coumarin | |
| 2H- | furo[2,3-H]-1-benzopyran-2-one |
| 2H- | furo[2,3-H][1]benzopyran-2-one |
| furo[2,3-h]chromen-2-one | |
| 2H- | furo[2,3-H]chromen-2-one |
| 4- | hydroxy-5-benzofuran acrylic acid gamma-lactone |
| 3-(4- | hydroxy-5-benzofuranyl)-2-propenoic acid gamma-lactone |
| 2- | propenoic acid, 3-(4-hydroxy-5-benzofuranyl)-, d-lactone |
| iso | psoralen |
