Articles:
2-methylpyrazine
Notes:
Flavouring agent. Present in many foods, e.g. bakery products, dairy products, meats, baked or French fried potato, roasted barley, cocoa, coffee, tea, roasted filbert, roasted pecan, peanut, soy products, rum and whisky
| Fragrance Demo Formulas Flavor Demo Formulas | ||
| CAS Number: | 109-08-0 | 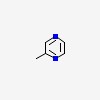 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 203-645-8 | |
| FDA UNII: | RVC6500U9C | |
| Nikkaji Web: | J1.501F | |
| Beilstein Number: | 0105778 | |
| MDL: | MFCD00006142 | |
| CoE Number: | 2270 | |
| XlogP3: | 0.20 (est) | |
| Molecular Weight: | 94.11682000 | |
| Formula: | C5 H6 N2 | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 761 2-methylpyrazine |
| DG SANTE Food Flavourings: | 14.027 2-methylpyrazine |
| FEMA Number: | 3309 2-methylpyrazine |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 109-08-0 ; 2-METHYLPYRAZINE |
Physical Properties:
| Appearance: | colorless to pale yellow clear liquid (est) |
| Assay: | 98.00 to 100.00 % |
| Food Chemicals Codex Listed: | Yes |
| Specific Gravity: | 1.01000 to 1.03000 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 8.404 to 8.571 |
| Specific Gravity: | 1.00700 to 1.03300 @ 25.00 °C. |
| Pounds per Gallon - est.: | 8.379 to 8.596 |
| Refractive Index: | 1.50400 to 1.50600 @ 20.00 °C. |
| Refractive Index: | 1.50100 to 1.50900 @ 20.00 °C. |
| Melting Point: | -29.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 135.00 °C. @ 761.00 mm Hg |
| Boiling Point: | 136.00 to 137.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 9.690000 mmHg @ 25.00 °C. (est) |
| Vapor Density: | 3.2 ( Air = 1 ) |
| Flash Point: | 122.00 °F. TCC ( 50.00 °C. ) |
| logP (o/w): | 0.210 |
| Soluble in: | |
| alcohol | |
| fixed oils | |
| water, 1000000 mg/L @ 20 °C (exp) | |
Organoleptic Properties:
| Odor Type: nutty | |
| nutty cocoa roasted chocolate peanut green | |
| Odor Description: at 1.00 % in dipropylene glycol. | nutty cocoa roasted chocolate peanut green |
| nutty brown nut skin musty earthy roasted | |
| Odor Description: | Nutty, brown, nut skin, musty, pyrazine and earthy with a slight roasted nuance Mosciano, Gerard P&F 19, No. 3, 51, (1994) |
| Flavor Type: nutty | |
| nutty brown roasted musty astringent | |
| Taste Description: at 75.00 ppm. | Nutty, brown, roasted, musty and astringent Mosciano, Gerard P&F 19, No. 3, 51, (1994) |
| Odor and/or flavor descriptions from others (if found). | |
| Bedoukian Research | |
| 2-METHYL PYRAZINE ≥99.0%, FCC, Kosher | |
| Odor Description: | A nutty, cocoa, roasted meat aroma |
| Taste Description: | nutty Used in coffee, cocoa, roasted meats, fresh bread and peanut butter flavors. |
| R C Treatt & Co Ltd | |
| 2-Methylpyrazine Halal, Kosher | |
| Odor Description: | nutty/roasted, peanut/chocolate, roast chicken |
| Taste Description: | nutty Used in flavours up to 10ppm, in applications such as baked goods, meat and cereal products, confectionery, beverages, and sauces. |
| Kingchem Laboratories | |
| 2 METHYL PYRAZINE | |
| Odor Description: | Roast, Coffee, Stir-fry eathnut |
| Pell Wall Perfumes | |
| Methyl Pyrazine | |
| Odor Description: | Nutty, cocoa, roasted, green. Powerful As with all pyrazines this is used in traces � Methyl Pyrazine at 0.1% in Ethanol has a nutty-chocolate odour (reminiscent of hazelnut chocolate spread) but also has a distinctive green-vegetable aspect. Very useful as part of an authentic chocolate or coffee accord, and can also contribute a nutty note to any gourmand fragrance or form part of a natural-smelling green complex. |
Cosmetic Information:
| None found |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 10 - Flammable. R 22 - Harmful if swallowed. R 36/37/38 - Irritating to eyes, respiratory system, and skin. S 02 - Keep out of the reach of children. S 16 - Keep away from sources of ignition - No Smoking. S 20/21 - When using do not eat, drink or smoke. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 36/37/39 - Wear suitable clothing, gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
gavage-rat LD50 1800 mg/kg (Moran et al., 1980) intraperitoneal-mouse LD50 1820 mg/kg Toxicology and Applied Pharmacology. Vol. 17, Pg. 244, 1970. oral-rat LD50 1800 mg/kg Drug and Chemical Toxicology. Vol. 3, Pg. 249, 1980. | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for 2-methyl pyrazine usage levels up to: | |||
| 0.1000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 17.00 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 7.00 (μg/capita/day) | ||
| Structure Class: | II | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 5 | |||
| Click here to view publication 5 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | 10.00000 | |
| beverages(nonalcoholic): | - | 10.00000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | 10.00000 | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | 10.00000 | |
| fruit ices: | - | 10.00000 | |
| gelatins / puddings: | - | 10.00000 | |
| granulated sugar: | - | - | |
| gravies: | - | 10.00000 | |
| hard candy: | - | 10.00000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | 10.00000 | |
| milk products: | - | 10.00000 | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | 10.00000 | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of pyrazine derivatives used as flavor ingredients. View pdf | |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to Flavouring Group Evaluation 17 (FGE.17): Pyrazine derivatives from chemical group 24 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) View page or View pdf | |
| Flavouring Group Evaluation 50 (FGE.50): Consideration of pyrazine derivatives evaluated by JECFA (57th meeting) structurally related to pyrazine derivatives evaluated by EFSA in FGE.17 (2005) (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 17, Revision 1 (FGE.17Rev1): Pyrazine derivatives from chemical group 24 - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) [1] View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 17, Revision 2 (FGE.17Rev2): Pyrazine derivatives from chemical group 24 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 50, Revision 1 (FGE.50Rev1): Consideration of pyrazine derivatives evaluated by JECFA (57th meeting) structurally related to pyrazine derivatives evaluated by EFSA in FGE.17Rev2 (2010) View page or View pdf | |
| Safety and efficacy of pyrazine derivatives including saturated ones belonging to chemical group 24 when used as flavourings for all animal species View page or View pdf | |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 109-08-0 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 7976 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 1993 |
| WGK Germany: | 3 |
| 2-methylpyrazine | |
| Chemidplus: | 0000109080 |
| RTECS: | UQ3675000 for cas# 109-08-0 |
References:
| 2-methylpyrazine | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 109-08-0 |
| Pubchem (cid): | 7976 |
| Pubchem (sid): | 134973513 |
| Flavornet: | 109-08-0 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEMBL: | View |
| HMDB (The Human Metabolome Database): | HMDB33112 |
| FooDB: | FDB011112 |
| Export Tariff Code: | 2933.99.8090 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
Potential Blenders and core components note
Potential Uses:
| almond toasted almond | FL | |
| bread | FL | |
| butter | FR | |
| chocolate | FR | |
| chocolate cocoa | FL | |
| coffee | FR | |
| green | FR | |
| meat roasted meat | FL | |
| peanut butter | FL | |
| potato | FL |
Occurrence (nature, food, other): note
| almond roasted almond Search Trop Picture | |
| almond seed Search Trop Picture | |
| asparagus Search Trop Picture | |
| beef Search PMC Picture | |
| bread white bread Search PMC Picture | |
| chicken Search PMC Picture | |
| coffee Search PMC Picture | |
| fenugreek seed Search Trop Picture | |
| fish Search PMC Picture | |
| guava fruit Search Trop Picture | |
| hazelnut Search Trop Picture | |
| kohlrabi stem Search Trop Picture | |
| lamb Search PMC Picture | |
| lavender oil spike spain @ 0.074% Data GC Search Trop Picture | |
| macadamia Search PMC Picture | |
| papaya fruit Search Trop Picture | |
| peanut Search Trop Picture | |
| pork Search PMC Picture | |
| potato chip Search PMC Picture | |
| rice Search Trop Picture | |
| rice cakes PbMd Search PMC Picture | |
| seafood Search PMC Picture | |
| shrimp Search PMC Picture | |
| tea black tea Search Trop Picture | |
| tea green tea Search Trop Picture | |
| tea leaf Search Trop Picture | |
| tomato Search Trop Picture |
Synonyms:
| methyl pyrazine | |
| 2 | methyl pyrazine |
| 2- | methyl pyrazine FCC |
| 2- | methyl-1,4-diazine |
| (±)-2- | methylpiperazine |
| 2- | methylpyrazine |
| pyrazine, 2- methyl | |
| pyrazine, 2-methyl- |



























