Articles:
1-benzo(b)pyrrole
Notes:
Constit. of several flower oils, esp. of Jasminum and Citrus spp. (Oleaceae) prod. of bacterial dec. of proteins. Flavouring ingredient. Also present in crispbread, Swiss cheese, Camembert cheese, wine, cocoa, black and green tea, rum, roasted filbert, rice bran, clary sage, raw shrimp and other foodstuffs
Indole is a major constituent of coal-tar, and the 220-260 �C distillation fraction is the main industrial source of the material. Indole and its derivatives can also be synthesized by a variety of methods. The main industrial routes start from aniline.; Indole is a solid at room temperature. Indole can be produced by bacteria as a degradation product of the amino acid tryptophan. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many flower scents (such as orange blossoms) and perfumes. It also occurs in coal tar.; Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an indole ring are called indoles. The most famous derivative is the amino acid tryptophan.; Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. It can be produced by bacteria as a degradation product of the amino acid tryptophan. It occurs naturally in human feces and has an intense fecal smell. At very low concentrations, however, it has a flowery smell, and is a constituent of many flower scents (such as orange blossoms) and perfumes. Natural jasmine oil, used in the perfume industry, contains around 2.5% of indole. Indole also occurs in coal tar. The participation of the nitrogen lone electron pair in the aromatic ring means that indole is not a base, and it does not behave like a simple amine.; The Leimgruber-Batcho indole synthesis is an efficient method of sythesizing indole and substituted indoles. Originally disclosed in a patent in 1976, this method is high-yielding and can generate substituted indoles. This method is especially popular in the pharmaceutical industry, where many pharmaceutical drugs are made up of specifically substituted indoles.; The name indole is a portmanteau of the words indigo and oleum, since indole was first isolated by treatment of the indigo dye with oleum.
| Fragrance Demo Formulas Flavor Demo Formulas | ||
| CAS Number: | 120-72-9 | 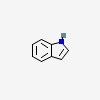 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 204-420-7 | |
| FDA UNII: | 8724FJW4M5 | |
| Nikkaji Web: | J2.920C | |
| Beilstein Number: | 0107693 | |
| MDL: | MFCD00005607 | |
| CoE Number: | 560 | |
| XlogP3: | 2.10 (est) | |
| Molecular Weight: | 117.15079000 | |
| Formula: | C8 H7 N | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 1301 indole |
| DG SANTE Food Flavourings: | 14.007 indole |
| FEMA Number: | 2593 indole |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 120-72-9 ; INDOLE |
| FDA Regulation: | |
| FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION Subpart F--Flavoring Agents and Related Substances Sec. 172.515 Synthetic flavoring substances and adjuvants. | |
Physical Properties:
| Appearance: | white to amber yellow crystalline solid powder (est) |
| Assay: | 97.00 to 100.00 % |
| Food Chemicals Codex Listed: | Yes |
| Melting Point: | 51.00 to 54.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 253.00 to 254.00 °C. @ 760.00 mm Hg |
| Congealing Point: | 51.10 °C. |
| Vapor Pressure: | 0.012200 mmHg @ 25.00 °C. |
| Flash Point: | > 230.00 °F. TCC ( > 110.00 °C. ) |
| logP (o/w): | 2.140 |
| Shelf Life: | 36.00 month(s) or longer if stored properly. |
| Storage: | store in cool, dry place in tightly sealed containers, protected from heat and light. |
| Soluble in: | |
| alcohol | |
| fixed oils | |
| propylene glycol | |
| water, 3560 mg/L @ 25 °C (exp) | |
| Insoluble in: | |
| glycerin | |
Organoleptic Properties:
| Odor Type: animal | |
| Odor Strength: | high , recommend smelling in a 1.00 % solution or less |
| Substantivity: | 400 hour(s) at 50.00 % in dipropylene glycol |
| animal floral naphthyl fecal | |
| Odor Description: at 1.00 % in dipropylene glycol. | animal floral moth ball fecal naphthelene Luebke, William tgsc, (1982) |
| pungent floral naphthyl fecal animal musty | |
| Odor Description: at 1.00 %. | Pungent, floral, slightly naphtha and mothball like with a fecal and animalic musty character Mosciano, Gerard P&F 26, No. 3, 80, (2001) |
| Flavor Type: animal | |
| animal fecal naphthyl earthy phenolic chemical | |
| Taste Description: at 0.30 - 2.00 ppm. | Animal, fecal, naphthyl, with earthy, perfumey, phenolic and chemical nuances Mosciano, Gerard P&F 26, No. 3, 80, (2001) |
| Odor and/or flavor descriptions from others (if found). | |
| Alfrebro | |
| INDOLE (EU NAT) | |
| Odor Description: | Floral on high dilution |
| Moellhausen | |
| INDOLE | |
| Odor Description: | penetrating, animal, faecal |
| Taste Description: | musky, faecal, cheese |
| PerfumersWorld | |
| Indole | |
| Odor Description: | strong moth ball naphthelene erogenic-floral animal narcotic-floral jasmine slightly musk fecal Blends-well-with - +Phenyl Acetic Acid +Mimosa |
| Pell Wall Perfumes | |
| Indole | |
| Odor Description: | Animalic, floral-heady, faecal, naphthelene / moth-balls Arctander writes extensively about this material; here are a couple of extracts: “Extremely diffusive and powerful odor, almost tarry-repulsive and choking when concentrated, but in concentrations lower than 0.1%. or in compositions, it shows powerful floral notes and pleasant radiation. Good tenacity, in spite of the volatility at room temperature.” |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents |
Suppliers:
| ACS International |
| Indole
Odor: animal fecal earthy musty |
| Operational Capabilities |
| Advanced Biotech |
| INDOLE 0.6% IN ETOH NATURAL |
| Advanced Biotech |
| INDOLE 1% IN ETOH NATURAL |
| Advanced Biotech |
| INDOLE 1% IN PG NATURAL |
| Advanced Biotech |
| INDOLE NATURAL
97% min. |
| Alfa Biotechnology |
| For experimental / research use only. |
| Indole 98% |
| Alfrebro |
| INDOLE (EU NAT)
Odor: Floral on high dilution |
| Alfrebro |
| INDOLE (EU NAT)
Odor: Floral on high dilution |
| Alfrebro |
| INDOLE NATURAL 1% IN ETHANOL
Odor: Floral on high dilution |
| Anhui Haibei |
| Indole |
| Augustus Oils |
| Indole |
| Services |
| Axxence Aromatic |
| INDOLE Natural
Kosher |
| Sustainability |
| Beijing Lys Chemicals |
| Indole |
| Berjé |
| Indole Crystals |
| Media |
| BOC Sciences |
| For experimental / research use only. |
| Indole 95% |
| BST Tianjin Co. |
| Indole |
| Creatingperfume.com |
| Indole |
| Diffusions Aromatiques |
| INDOLE |
| EMD Millipore |
| For experimental / research use only. |
| Indole |
| Ernesto Ventós |
| INDOLE, NATURAL |
| Ernesto Ventós |
| INDOLE
Odor: FECAL IF CONC., FLORAL IF DILUTED |
| Excellentia International |
| Indole Natural |
| ExtraSynthese |
| For experimental / research use only. |
| Indole |
| Global Essence |
| Indole |
| Hermitage Oils |
| Indole 1% Natural Isolate
Odor: characteristic Use: Eleonora Scalseggi has this to say “Often called “magic” indole is indeed a very fascinating material whose importance in perfumery cannot be overstated. Depending on its concentration it can be terribly repulsive or immensely seductive, featuring in the aroma profile of some of the most bewitching floral scents on earth like jasmines and orange blossoms as well as in excrements. Indole is the seductive weapon devised by the most intoxicating night flowers to attract pollinators � usually moths. Funnily enough traces of indole in the aroma profile of a flower make it irresistible for humans too, capable of transforming a delicate, clean yet insignificant floral scent into a narcotic elixir. |
| Indenta Group |
| Indole |
| Indis NV |
| For experimental / research use only. |
| Indole 99% |
| Indukern F&F |
| INDOL CRYST.
Odor: ANIMAL, FLORAL |
| K.L. Koh Enterprise |
| INDOLE |
| Lluch Essence |
| INDOLE CRYST. |
| Lluch Essence |
| INDOLE NATURAL |
| M&U International |
| INDOLE |
| Moellhausen |
| INDOLE 10%DPG |
| Moellhausen |
| INDOLE
Odor: penetrating, animal, faecal Flavor: musky, faecal, cheese |
| Natural Advantage |
| Indole Nat, 10% in OH
Flavor: creamy, floral, sweet |
| Riverside Aromatics LTD.is the exclusive distributor for Europe in UK for any non-US based inquiries |
| O'Laughlin Industries |
| INDOLE |
| Pell Wall Perfumes |
| Indole
Odor: Animalic, floral-heady, faecal, naphthelene / moth-balls Use: Arctander writes extensively about this material; here are a couple of extracts: “Extremely diffusive and powerful odor, almost tarry-repulsive and choking when concentrated, but in concentrations lower than 0.1%. or in compositions, it shows powerful floral notes and pleasant radiation. Good tenacity, in spite of the volatility at room temperature.” |
| Penta International |
| INDOLE FCC GRADE |
| Penta International |
| INDOLE NATURAL (NEAT) |
| Penta International |
| INDOLE NATURAL IN ETHYL ALCOHOL |
| Penta International |
| INDOLE |
| PerfumersWorld |
| Indole 10% in DPG |
| PerfumersWorld |
| Indole
Odor: strong moth ball naphthelene erogenic-floral animal narcotic-floral jasmine slightly musk fecal Use: Blends-well-with - +Phenyl Acetic Acid +Mimosa |
| Prodasynth |
| INDOLE
(> 99%) Odor: FECAL IF CONC., FLORAL IF DILUTED |
| Reincke & Fichtner |
| Indole |
| Sigma-Aldrich |
| Indole, ≥99%, FG
Odor: butter; cheese; chocolate; grape; honey; jasmine; musty; floral; fatty; vanilla; animal; earthy; vegetable; wine-like; fishy |
| Certified Food Grade Products |
| Sigma-Aldrich |
| Indole, natural, ≥97%, FG
Odor: floral; pungent; animalic |
| SRS Aromatics |
| INDOLE |
| TCI AMERICA |
| For experimental / research use only. |
| Indole >99.0%(GC) |
| The John D. Walsh Company |
| Indole |
| The Lermond Company |
| INDOLE |
| Vigon International |
| Indole
Odor: UNPLEASANT ODOR IN HIGH CONCENTRATIONS, BUT FLORAL IN HIGHER DILUTIONS |
| Zanos |
| Indole
Odor: Powerful and harsh odour, with a-jasmine character on dilution |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn N - Harmful, Dangerous for the environment. | |
|
R 21/22 - Harmful in contact with skin and if swallowed. R 37/38 - Irritating to respiratory system and skin. R 41 - Risk of serious damage to eyes. R 50/53 - Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. S 02 - Keep out of the reach of children. S 20/21 - When using do not eat, drink or smoke. S 22 - Do not breath dust. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 36/37/39 - Wear suitable clothing, gloves and eye/face protection. S 60 - This material and its container must be disposed of as hazardous waste. S 61 - Avoid release to the environment. Refer to special instructions/safety data sheet. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| Acute toxicity, Oral (Category 4), H302 Skin irritation (Category 2), H315 Serious eye damage (Category 1), H318 Specific target organ toxicity - single exposure (Category 3), Respiratory system, H335 Acute aquatic toxicity (Category 1), H400 Chronic aquatic toxicity (Category 1), H410 | |
| GHS Label elements, including precautionary statements | |
| Pictogram |    |
| Signal word | Danger |
| Hazard statement(s) | |
| H302 - Harmful if swallowed H311 - Toxic in contact with skin H315 - Causes skin irritation H318 - Causes serious eye damage H335 - May cause respiratory irritation H400 - Very toxic to aquatic life H410 - Very toxic to aquatic life with long lasting effects | |
| Precautionary statement(s) | |
| P261 - Avoid breathing dust/fume/gas/mist/vapours/spray. P264 - Wash skin thouroughly after handling. P270 - Do not eat, drink or smoke when using this product. P271 - Use only outdoors or in a well-ventilated area. P273 - Avoid release to the environment. P280 - Wear protective gloves/protective clothing/eye protection/face protection. P301 + P312 - IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302 + P352 - IF ON SKIN: wash with plenty of soap and water. P304 + P340 - IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305 + P351 + P338 - IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P310 - Immediately call a POISON CENTER or doctor/physician. P330 - Rinse mouth. P332 + P313 - IF SKIN irritation occurs: Get medical advice/attention. P361 - Remove/Take off immediately all contaminated clothing. P391 - Collect spillage. Hazardous to the aquatic environment P403 + P233 - Store in a well-ventilated place. Keep container tightly closed. P405 - Store locked up. P501 - Dispose of contents/ container to an approved waste disposal plant. | |
| Human Experience: | |
| 1 % solution: non-sensitising. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 [sex: M] 1000 mg/kg (Smyth et al., 1962) intraperitoneal-mouse LD50 117 mg/kg Yakugaku Zasshi. Journal of Pharmacy. Vol. 94, Pg. 1620, 1974. oral-mouse LDLo 1070 mg/kg Archives of Environmental Contamination and Toxicology. Vol. 14, Pg. 111, 1985. oral-rat LD50 1000 mg/kg American Industrial Hygiene Association Journal. Vol. 23, Pg. 95, 1962. | |
| Dermal Toxicity: | |
|
skin-rabbit LD50 790 mg/kg American Industrial Hygiene Association Journal. Vol. 23, Pg. 95, 1962. subcutaneous-mouse LD50 225 mg/kg Klinische Wochenscrift. Vol. 35, Pg. 504, 1957. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for indole usage levels up to: | |||
| 2.0000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 26.00 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 10.00 (μg/capita/day) | ||
| Structure Class: | I | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 3 | |||
| Click here to view publication 3 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | 0.58000 | |
| beverages(nonalcoholic): | - | 0.26000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | 0.28000 | |
| fruit ices: | - | 0.28000 | |
| gelatins / puddings: | 0.02000 | 0.40000 | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | 0.50000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 24 (FGE.24): Pyridine, pyrrole, indole and quinoline derivatives from chemical group 28 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) View page or View pdf | |
| Pyridine, pyrrole, indole and quinoline derivatives from chemical group 28 Flavouring Group Evaluation 24, Revision 1 - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 77 (FGE77) [1] - Consideration of Pyridine, Pyrrole and Quinoline Derivatives evaluated by JECFA (63rd meeting) structurally related to Pyridine, Pyrrole, Indole and Quinoline Derivatives evaluated by EFSA in FGE.24Rev1 (2008) View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 77, Revision 2 (FGE.77Rev2): Consideration of Pyridine, Pyrrole and Quinoline Derivatives evaluated by JECFA (63rd meeting) structurally related to Pyridine, Pyrrole, Indole and Quinoline Derivatives evaluated by EFSA in FGE.24Rev2 (2013) View page or View pdf | |
| Safety and efficacy of pyridine and pyrrole derivatives belonging to chemical group 28 when used as flavourings for all animal species View page or View pdf | |
| Scientific opinion on flavouring group evaluation 77, revision 3 (FGE.77Rev3): consideration of pyridine, pyrrole and quinoline derivatives evaluated by JECFA (63rd meeting) structurally related to pyridine, pyrrole, indole and quinoline derivatives evaluated by EFSA in FGE.24Rev2 View page or View pdf | |
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Daily Med: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| Carcinogenic Potency Database: | Search |
| EPA Substance Registry Services (TSCA): | 120-72-9 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 798 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 2811 |
| WGK Germany: | 1 |
| 1H-indole | |
| Chemidplus: | 0000120729 |
| RTECS: | NL2450000 for cas# 120-72-9 |
References:
| 1H-indole | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 120-72-9 |
| Pubchem (cid): | 798 |
| Pubchem (sid): | 134975207 |
| Flavornet: | 120-72-9 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| Metabolomics Database: | Search |
| KEGG (GenomeNet): | C00463 |
| HMDB (The Human Metabolome Database): | HMDB00738 |
| FooDB: | FDB012008 |
| Export Tariff Code: | 2933.90.0500 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| Formulations/Preparations: •found in neroli oil in trace quantities approx 0.1% •constitutes 2.5% of jasmine oil •technical, cp, fcc | |
Potential Blenders and core components note
Potential Uses:
| acacia | FR | |
| carnation | FR | |
| chocolate cocoa | FL | |
| cinq fleurs forvil | ||
| citrus | FR | |
| civet | FR | |
| coffee | FR | |
| crabapple blossom | FR | |
| egg | FL | |
| floral | FR | |
| gardenia | FR | |
| grape concord grape | FR | |
| honey | FR | |
| honeysuckle | FR | |
| jasmin | FR | |
| jonquil | FR | |
| lilac | FR | |
| lily of the valley | FR | |
| lotus | FR | |
| lumiere | ||
| lux beauty shower soap | ||
| mimosa | FR | |
| narcissus | FR | |
| neroli | FR | |
| olive | FL | |
| orange bitter orange peel | FL/FR | |
| orange blossom | FR | |
| orchid | FR | |
| oriental | FR | |
| rose | FR | |
| tobacco | FR | |
| tuberose | FR | |
| vanilla | FR | |
| violet | FR | |
| vol de nuit | ||
| wallflower | FR |
Occurrence (nature, food, other): note
| bergamot oil @ 0.000-0.009% Data GC Search Trop Picture | |
| bergamot oil @ trace% Data GC Search Trop Picture | |
| bonito dried bonito Search PMC Picture | |
| butter Search PMC Picture | |
| champaca absolute @ 2.90% Data GC Search Trop Picture | |
| champaca concrete @ 4.00% Data GC Search Trop Picture | |
| coffee roasted coffee Search PMC Picture | |
| corn shoot Search Trop Picture | |
| couroupita guianensis aubl. flower oil brazil @ 0.20% Data GC Search Trop Picture | |
| egg Search PMC Picture | |
| elder black elder leaf oil Search Trop Picture | |
| fig fruit Search Trop Picture | |
| fish Search PMC Picture | |
| hyacinthus orientalis absolute @ 0.05-0.08% Data GC Search Trop Picture | |
| jasmin Search PMC Picture | |
| jasmin absolute concrete egypt @ 3.84% Data GC Search Trop Picture | |
| jasmin absolute concrete india @ 1.07-1.85% Data GC Search Trop Picture | |
| jasmin absolute concrete italy @ 1.39% Data GC Search Trop Picture | |
| jasmin oil italy @ 4.21% Data GC Search Trop Picture | |
| jonquil Search Trop Picture | |
| kohlrabi stem Search Trop Picture | |
| lecythis usitata miers. var. paraensis (ducke) r. kunth. flower oil brazil @ 0.40% Data GC Search Trop Picture | |
| lemon Search Trop Picture | |
| lime Search Trop Picture | |
| malt Search PMC Picture | |
| mandarin Search Trop Picture | |
| michelia alba flower absolute @ 0.02% Data GC Search Trop Picture | |
| michelia champaca flower absolute @ 7.20% Data GC Search Trop Picture | |
| mikan peel oil @ trace% Data GC Search Trop Picture | |
| mustard white mustard Search Trop Picture | |
| narcissus absolute @ 1.51% Data GC Search Trop Picture | |
| narcissus absolute @ 6.30% Data GC Search Trop Picture | |
| neroli Search PMC Picture | |
| neroli oil CO2 extract @ 0.45% Data GC Search Trop Picture | |
| olive oil Search Trop Picture | |
| orangeflower absolute morocco @ 2.6-9.9% Data GC Search Trop Picture | |
| orangeflower water absolute @ 0.0-3.2% Data GC Search Trop Picture | |
| petitgrain combava oil @ trace% Data GC Search Picture | |
| rice black rice cooked PbMd Search PMC Picture | |
| robinia pseudacacia Search Trop Picture | |
| rum Search PMC Picture | |
| tea leaf Search Trop Picture | |
| thyme oil wild or creeping france @ 0.09% Data GC Search Trop Picture | |
| tobacco burley tobacco Search Picture | |
| wallflower Search Picture | |
| wine Search Picture | |
| ylang ylang oil CO2 extract @ 0.13% Data GC Search Trop Picture |
Synonyms:
| azaindole | |
| benzazole | |
| 1- | benzazole |
| 1- | benzo(b)pyrrole |
| benzo[b]pyrrole | |
| 1- | benzol(b)pyrrol |
| benzopyrrole | |
| 2,3- | benzopyrrole |
| 1-aza | indene |
| indol cryst. | |
| 1H- | indole |
| indole 0.6% in ETOH natural | |
| indole 1% in ETOH natural | |
| indole 1% in PG natural | |
| indole crystals | |
| indole FCC | |
| indole natural | |
| ketole |






























